Non-invasive monitoring of blood metabolite levels
A metabolite, blood technology, applied in the field of non-invasive monitoring, can solve problems such as compensation difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0064] Example 1: Identification of organizational layers
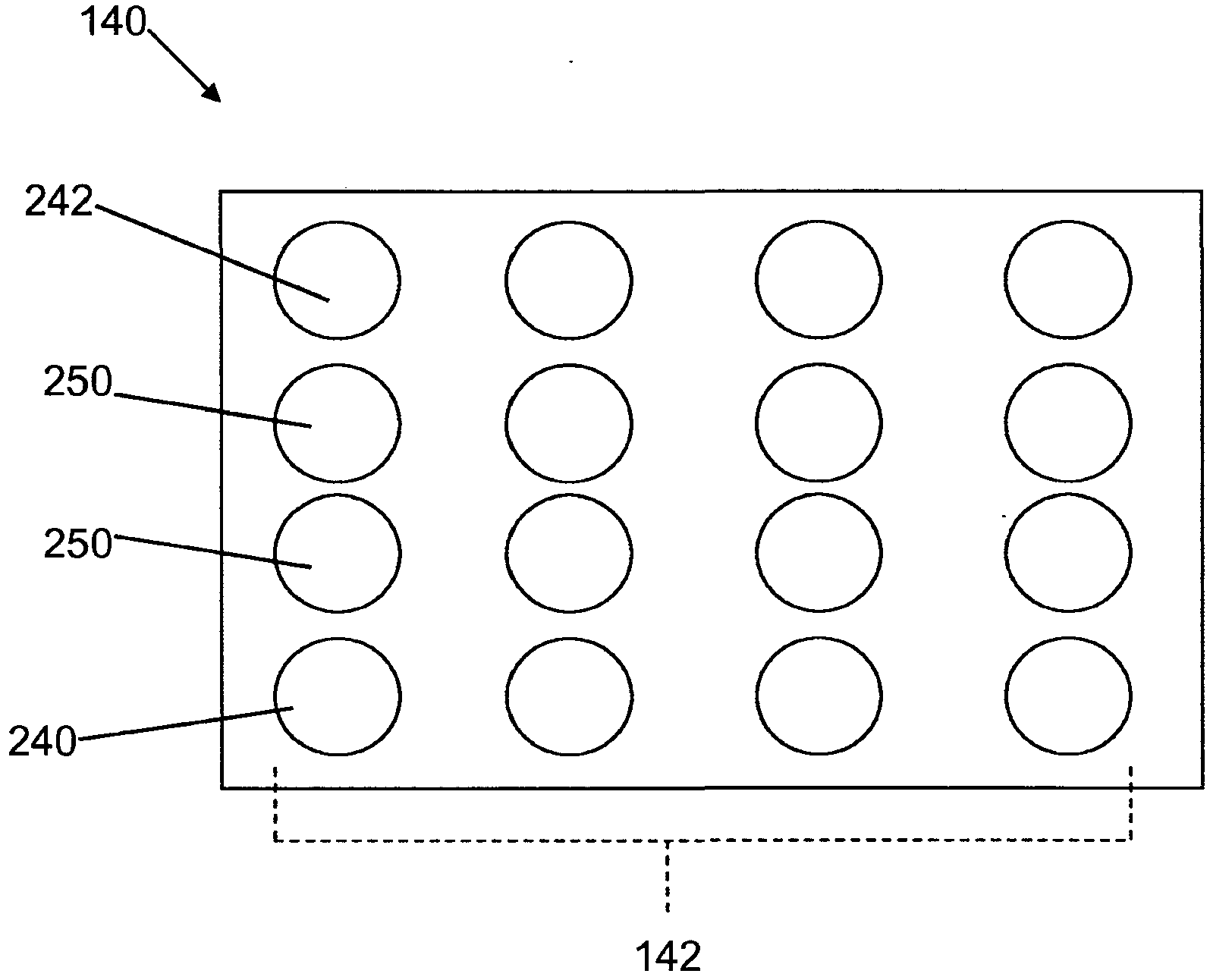
[0065] The following is achieved by using the Figure 4 An illustrative example of experimental results obtained with sensor array 143 of glucose monitor 140 . All sensors used in this experiment are disposable electrode( is a registered trademark of BIOPAC Systems, Inc., Goleta, California), and each electrode has a diameter of 10.5 mm. Figure 10 A schematic side view of the sensor array 143 as used in this experiment is shown. As described herein, each electrode in sensor array 143 is assigned a number (1-8). The sensor array 143 is configured such that the distance between the electrodes (center to center) is X and the distance from the center of electrode 1 to the center of electrode 8 is 7X (equal spacing between electrodes). In this example, measurements were obtained using a set of four electrodes, including one current-sending electrode, one current-receiving electrode, and two voltage-sensing electrod...
example 2
[0067] Example 2: Tissue Volume Removal
[0068] Figure 12 A conceptual model of the measured tissue volume and its measured impedance is shown. In this model, Z A representation mode A The measured impedance measure and volume of Z, and Z E representation mode E Measured impedance measurements and volumes of . In this test, the pattern E The distance between the electrodes in is the mode A Twice the distance between the electrodes in (2X to X). So, when starting from Z E Remove Z A The effect of (determining Z A and Z E difference between), Z1 is equal to Z A with Z A in series, so Z1=Z A +Z A (Equation 1 below). Z E is the parallel combination of Z1 and Z2, thus the parallel combination equation is Z E =(Z1Z2) / (Z1+Z2). Substitute into Z1 to get, Z E =(Z A +Z A )*Z2 / ((Z A +Z A )+Z2), where Z1 is the impedance value of the tissue from the surface to a depth of X and Z2 is the impedance value of the tissue from a depth of X to a depth of 2X. In one tes...
example 3
[0072] Example 3: Tissue volume differentiation
[0073]Further tests are performed to determine the difference between the readings from the patient's epidermis, and one of the dermis and subcutaneous layers. Electromagnetic impedance readings were measured from a standard sodium chloride solution of 140 mmol / L using sensor array 143 . Given the uniform volume of NaCl solution, it is empirically derived that at a single frequency each electrode pair ( Figure 11 ) between the measured volumes, whereby:
[0074] Z I =k IG Z G =k IC Z C (Equation 3)
[0075] Z G =k GC Z C (equation 4)
[0076] where Z is the measured mode ( I , G , C ) impedance and use 140mmol / L standard sodium chloride solution to calculate K IG 、K IC and K GC . To test whether these empirically derived relationships hold for animal tissues, two tests were done. Test A was performed on animal muscle tissue having a thickness of 35 mm, where the k value for homogeneous muscle ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com