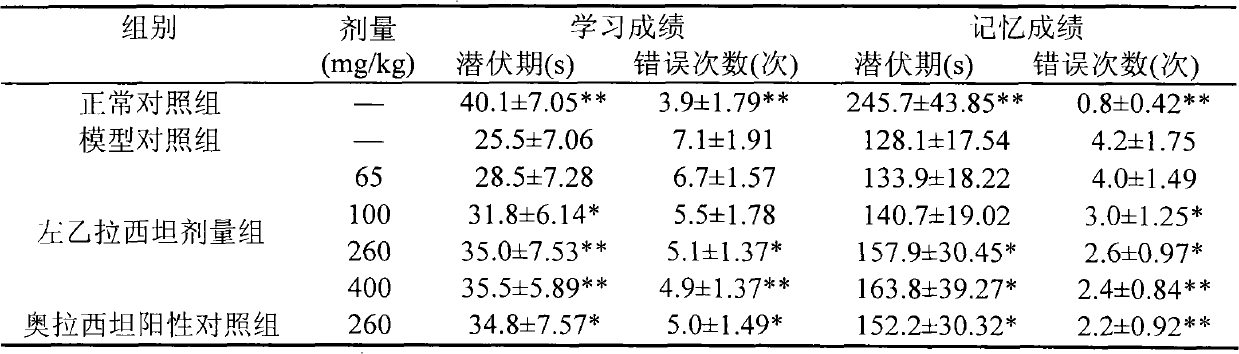
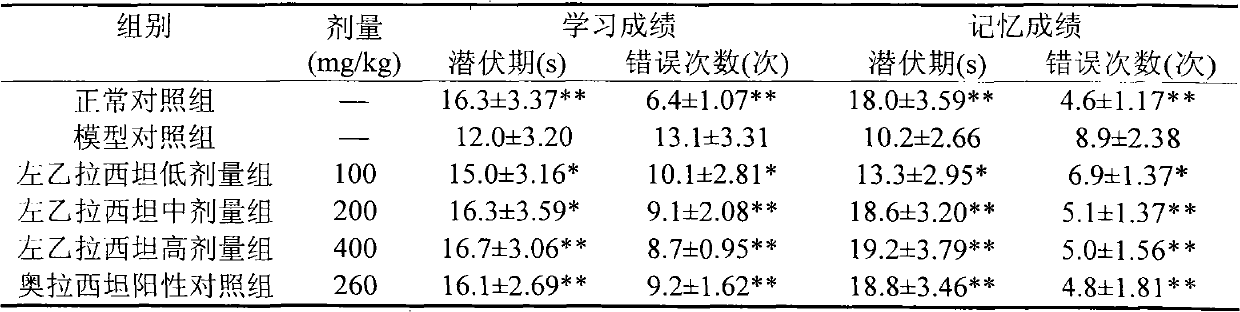
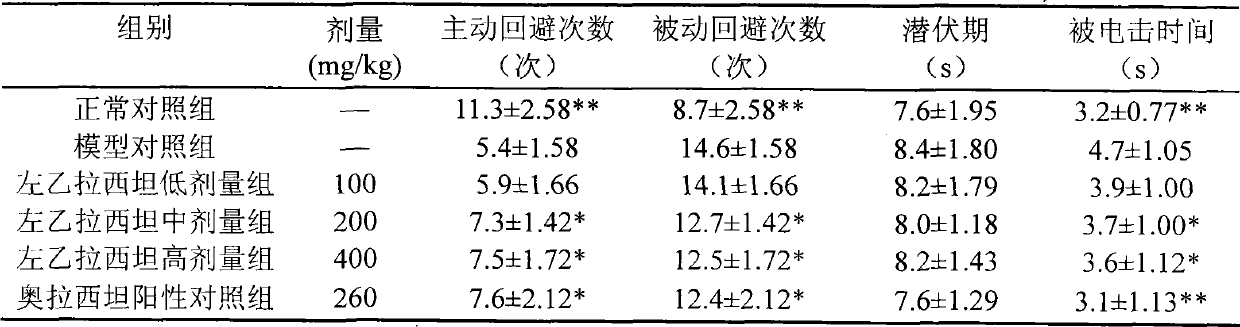
Application of levetiracetam injection in preparation of nootropic drugs
A technology for injections and uses, applied in the field of medicine, can solve problems such as inconvenience in use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Weigh 100g of levetiracetam, 150g of glucose, and 500ml of water for injection to dissolve in the dilute tank, control at 40-50°C, and stir until completely dissolved in 500ml of water for injection, and cool the solution to 20°C. Add activated carbon to the prepared solution for decolorization, then remove the activated carbon by filtration, add the prepared citric acid and sodium citrate buffer solution to adjust the pH value of the solution to 4.0, and then add water for injection to 5000ml , filling and sealing the bag, and sterilizing the filled and sealed intermediate product at 105°C for 30 minutes to obtain levetiracetam injection, and the metal ion concentration of the levetiracetam injection was detected to be 0.5PPM.
Embodiment 2
[0068] Weigh 100g of levetiracetam, 250g of glucose, and 800ml of water for injection to dissolve in the dilute tank, control at 50-60°C, and stir until completely dissolved in 800ml of water for injection, and cool the solution to 20°C. Add activated carbon to the prepared solution for decolorization, then remove the activated carbon by filtration, add the prepared citric acid and sodium citrate buffer solution to adjust the pH value of the solution to 4.2, then add water for injection to 7000ml , and then use a 0.02μm ultrafiltration membrane to perform ultrafiltration, and then centrifuge in a centrifuge at 2500r / min for 3min to remove insoluble matter, add water for injection until the volume of the injection reaches 7000ml, and fill and seal the bag at 105°C The intermediate product was sterilized for 30min, filled and sealed into bags to obtain levetiracetam injection, and the metal ion concentration of the injection was detected to be 0.8PPM.
Embodiment 3
[0070] Weigh 100g of levetiracetam, 550g of glucose, and 1000ml of water for injection and dissolve it in a dilute tank, control it at 60-70°C, and stir until it is completely dissolved in 1000ml of water for injection, and cool the solution to 20°C. Add activated carbon to the prepared solution for decolorization, then remove the activated carbon by filtration, add the prepared citric acid and sodium citrate buffer solution to adjust the pH value of the solution to 4.5, and then add water for injection to 10000ml , and then use a 0.02μm ultrafiltration membrane to perform ultrafiltration, and then centrifuge in a centrifuge at 2500r / min for 3min to remove insoluble matter, add water for injection until the volume of the injection reaches 10000ml, and fill and seal the bag at 105°C The intermediate product was sterilized for 30min, filled and sealed into bags to obtain levetiracetam injection, and the metal ion concentration of the injection was detected to be 0.5PPM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com