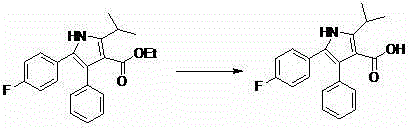
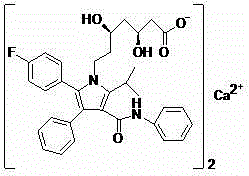
Method for preparing atorvastatin calcium
A technology of atorvastatin calcium and compounds, which is applied in the field of preparation of atorvastatin calcium, can solve the problems of high cost of atorvastatin calcium, high cost of compound 3, and difficult synthesis, and achieves cheap raw materials, mild reaction conditions, and easy separation simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0043]In order to make the technical means, creative features, work flow, and use methods of the present invention achieve the purpose and effect easily understood, the present invention will be further described below in conjunction with specific embodiments.
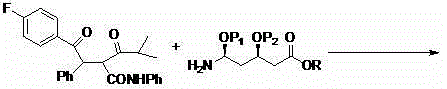
[0044] Synthesis of Compound II:
[0045] Add 608 grams of benzene (8.00mol) into the reaction flask, stir, cool to 0°C, add 160.2 grams of anhydrous aluminum trichloride (1.20mol), and slowly add 172.5 grams of 4-fluorophenylethyl Acyl chloride (1.00mol), after the dropwise addition, return to room temperature and react for 1 hour, then raise the temperature to 50°C and react for 10 hours. After the reaction, the reaction solution is cooled to room temperature, poured into 1200 ml of ice water, and added with 200 ml of 3N hydrochloric acid while stirring , the reaction solution was separated, the aqueous layer was extracted with benzene, the organic layers were combined, washed successively with 3N hydrochloric acid, ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap