Diaryl compound and preparation method thereof
A compound, biaryl technology, applied in the field of organic synthesis, can solve problems such as application limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
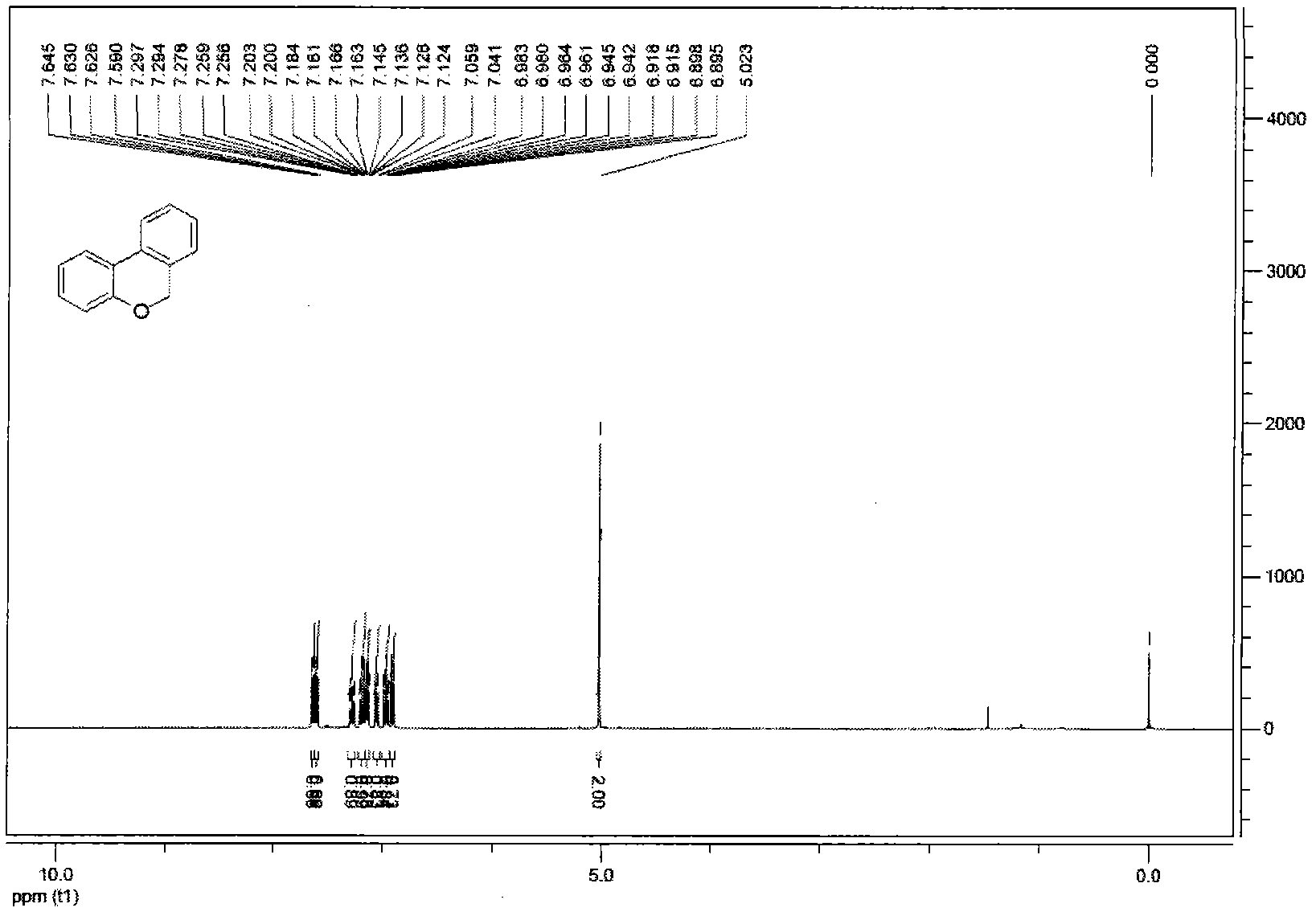
[0045] Under the reaction conditions of nitrogen gas, Pd(OAc) was successively loaded into the reaction flask 2 (4mol%, 0.9mg), PPh 3 (8mol%, 2.1mg), compound 1a (0.1mmol, 30mg), K 2 CO 3 (0.12mmol, 17mg), solvent N-methylpyrrolidone (0.5mL). Then the system was stirred in the air at 75°C for about 12 hours, and the reaction was quenched by adding HCl (1N, 5mL), extracted with ethyl acetate (15mL×3), then dried with anhydrous sodium sulfate, and then washed with 100- Adsorbed on 200-mesh silica gel, and then washed through a 300-400-mesh silica gel column to obtain product 2a with a yield of 96%. oily liquid; 1 H NMR (400MHz, CDCl 3)δ5.02(s, 2H), 6.91(dd, J=8.1, 1.2Hz, 1H), 6.96(dt, J=7.5, 1.2Hz, 1H), 7.05(d, J=7.4Hz, 1H), 7.12-7.20(m, 2H), 7.28(t, J=7.6Hz, 1H), 7.60(d, J=7.7Hz, 1H), 7.64(dd, J=7.7, 1.6Hz, 1H); IR(KBr ): 3179, 1484, 1400, 1244, 1120, 1017, 755, 688cm -1 ; MS (EI) m / z: 182 (M + ), 181, 152, 127, 115, 91, 76. The above data prove that the t...
Embodiment 2
[0047]
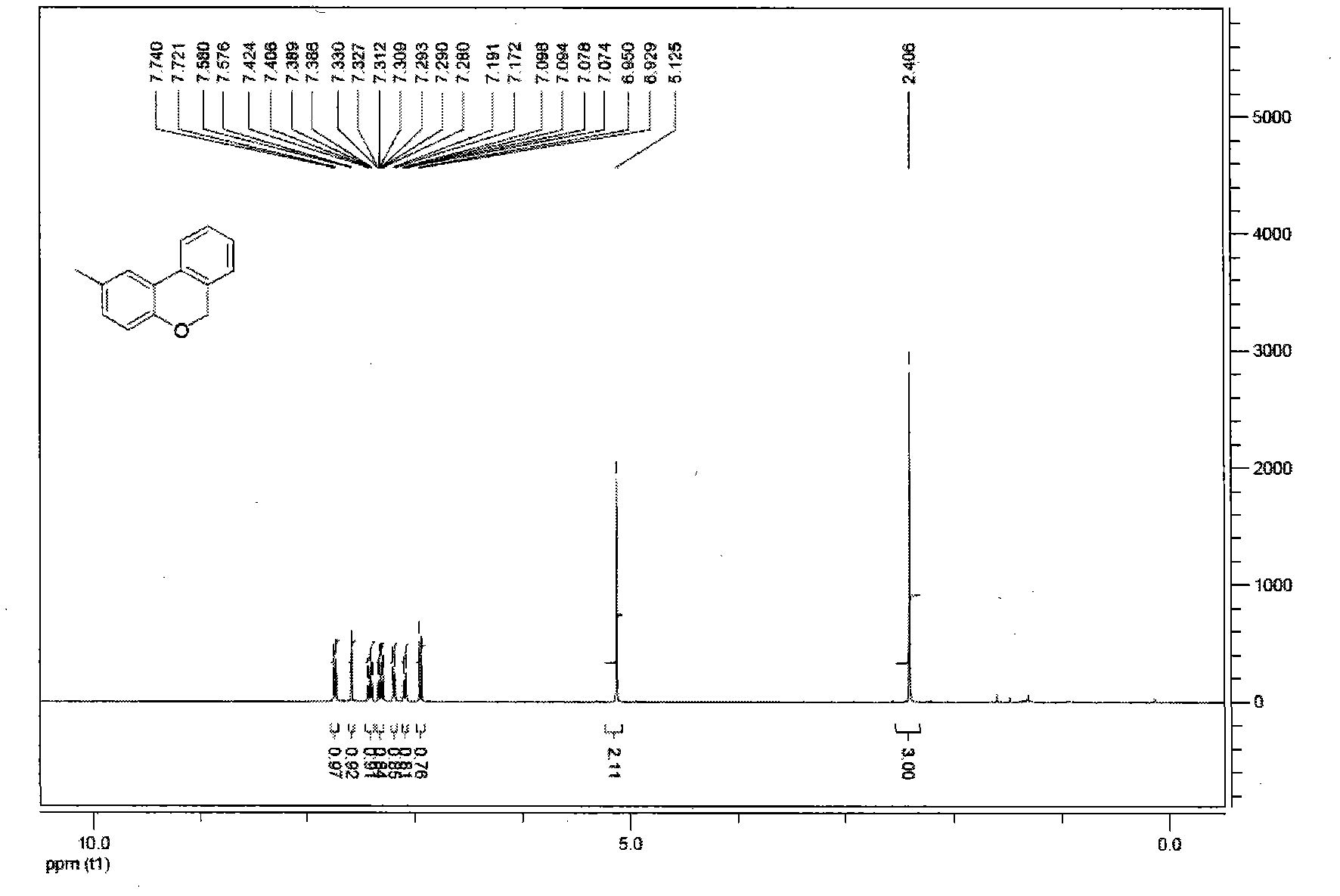
[0048] Under the reaction conditions of nitrogen gas, Pd(OAc) was successively loaded into the reaction flask 2 (4mol%, 0.9mg), PPh 3 (8mol%, 2.1mg), compound 1b (0.1mmol, 32mg), K 2 CO 3 (0.12mmol, 17mg), solvent N-methylpyrrolidone (0.5mL). Then the system was stirred in the air at 75°C for about 12 hours, and the reaction was quenched by adding HCl (1N, 5mL), extracted with ethyl acetate (15mL×3), then dried with anhydrous sodium sulfate, and then washed with 100- Adsorbed on 200-mesh silica gel, and then washed through a 300-400-mesh silica gel column to obtain product 2b with a yield of 99%. oily liquid; 1 H NMR (400MHz, CDCl 3 )δ2.41(s, 3H), 5.12(s, 2H), 6.94(d, J=8.2Hz, 1H), 7.09(dd, J=8.2, 1.6Hz, 1H), 7.18(d, J=7.5 Hz, 1H), 7.31(dt, J=7.5, 1.1Hz, 1H), 7.41(t, J=7.6, 7.6Hz, 1H), 7.58(d, J=1.6Hz, 1H), 7.73(d, J =7.7Hz, 1H); IR (KBr): 3030, 2960, 2920, 2842, 1497, 1250, 816, 770cm -1 ; MS (EI) m / z: 197 (M + ), 196, 195, 181, 165, 139, 115, 97. The ab...
Embodiment 3
[0050]
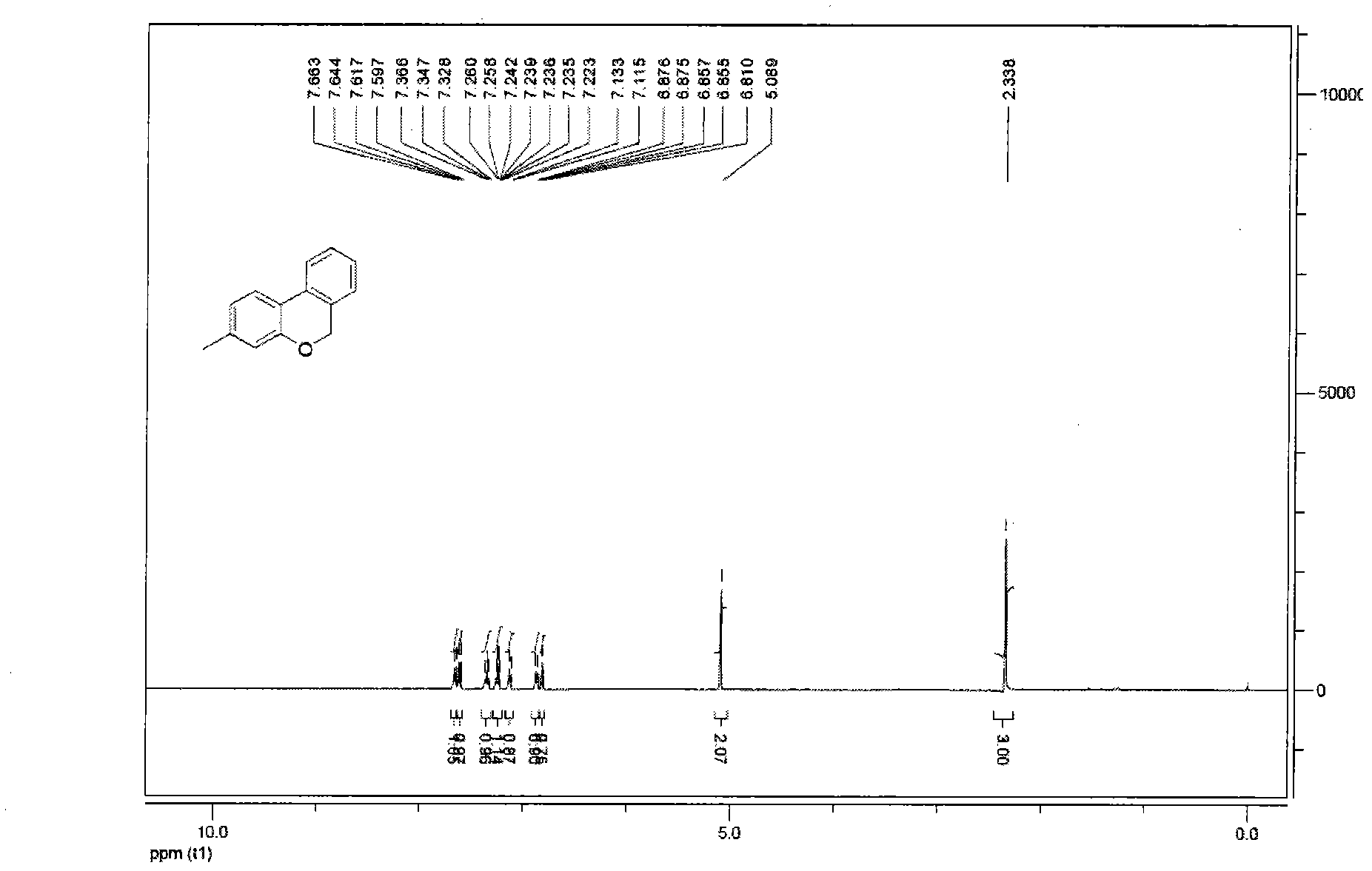
[0051] Under the reaction conditions of nitrogen gas, Pd(OAc) was successively loaded into the reaction flask 2 (4mol%, 0.9mg), PPh 3 (8mol%, 2.1mg), compound 1c (0.1mmol, 32mg), K 2 CO 3 (0.12mmol, 17mg), solvent N-methylpyrrolidone (0.5mL). Then the system was stirred in the air at 75°C for about 12 hours, and the reaction was quenched by adding HCl (1N, 5mL), extracted with ethyl acetate (15mL×3), then dried with anhydrous sodium sulfate, and then washed with 100- Adsorbed on 200-mesh silica gel, and then washed through a 300-400-mesh silica gel column to obtain product 2c with a yield of 92%. oily liquid; 1 H NMR (400MHz, CDCl 3 )δ2.34(s, 3H), 5.09(s, 2H), 6.81(s, 1H), 6.87(d, J=7.8Hz, 1H), 7.12(d, J=7.4Hz, 1H), 7.22- 7.26(m, 1H), 7.35(t, J=7.6Hz, 1H), 7.61(d, J=7.9Hz, 1H), 7.65(d, J=7.7Hz, 1H); IR(KBr): 3026, 2965, 2844, 1618, 1492, 1158, 1030, 770cm -1 ; MS (EI) m / z: 197 (M + ), 196, 195, 181, 165, 139, 115, 97. The above data prove that the target ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com