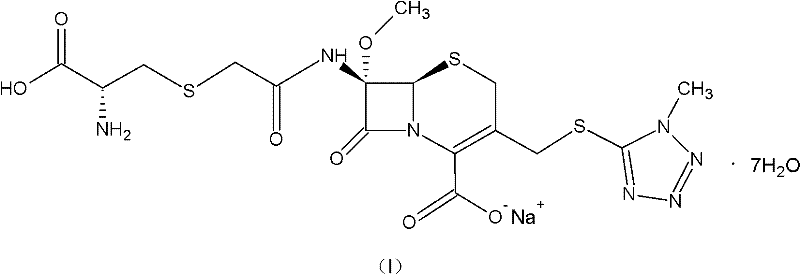
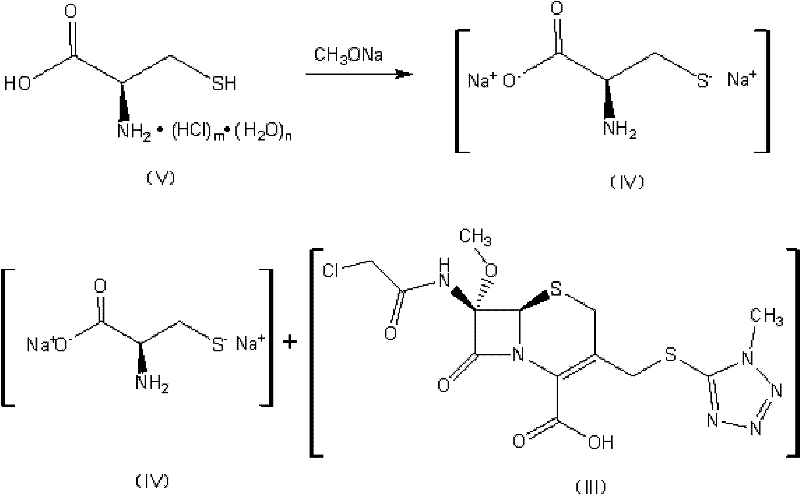
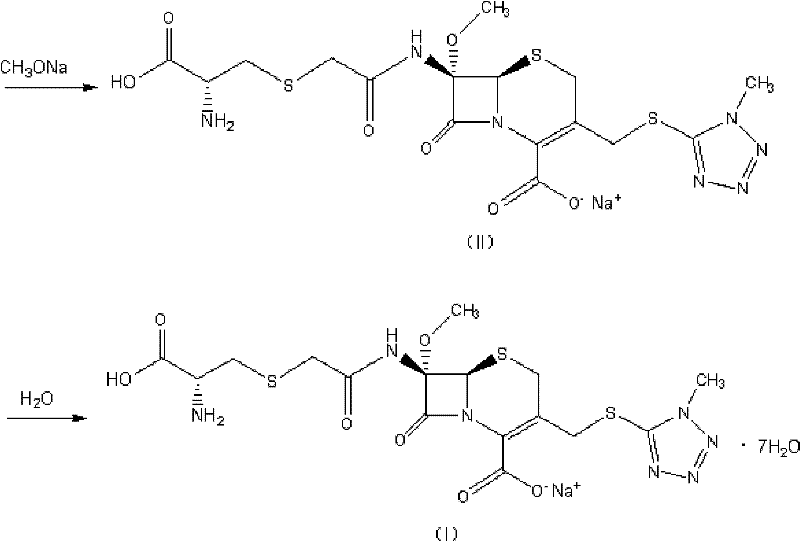
Preparation method of cefminox sodium
A technology of cefminox sodium and cefminox sodium, which is applied in the direction of organic chemistry, can solve the problems of affecting product quality and yield, high price, and unavoidable hydrolysis, so as to improve product purity, increase product yield, and inhibit The effect of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A preparation method for cefminox sodium, comprising the steps of:
[0045] (1) Add 20.0 g of anhydrous D-cysteine to 200 ml of methanol to prepare D-cysteine-methanol suspension; Add 90.0 g of sodium methoxide solution with a concentration of 30 wt % to the cystine-methanol suspension, and stir for 20 minutes to prepare a solution containing D-cysteine sodium thiolate (the structural compound of formula (IV));
[0046] (2) 1 L of ethyl acetate solution containing the compound of the formula (III) structure (43.5 g of the compound containing the formula (III) structure, the specific impurity IMP detected by HPLC is 0.65% (254nm, detected by the area normalization method, the same below )), concentrated under reduced pressure at 30°C and a pressure of -0.1MPa until the concentration of the compound of the formula (III) structure was 500g / L, cooled to 10°C, and added 300ml of methanol to obtain the compound containing the formula (III) structure The solution of the c...
Embodiment 2
[0055] Preparation method as described in Example 1, the difference is that,
[0056] In step (3), directly filter without adding ethyl acetate 600ml.
[0057] The weight of the obtained cefminox sodium heptahydrate is 53.5g, the yield is 79.5%, the product content is 99.1%, the HPLC purity is 99.5%, and the specific impurity IMP is 0.04%.
[0058] The obtained product was confirmed for its structure, and the data result was the same as the structure confirmed data of Example 1.
Embodiment 3
[0060] Preparation method as described in Example 1, the difference is that,
[0061] Methanol in the step (1) and step (2) is replaced by dimethyl sulfoxide.
[0062] The weight of the obtained cefminox sodium heptahydrate is 58.6g, the molar yield is 86.8%, the product content is 98.9%, the HPLC purity is 99.2%, and the specific impurity IMP is 0.07%.
[0063] The obtained product was confirmed for its structure, and the data result was the same as the structure confirmed data of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com