Monoglucoside injection of cycloastragenol and preparation method and application of injection
A technology of monoglucoside and cycloastragenol, which is applied in the field of pharmaceutical preparations, can solve the problems of unsatisfactory pharmacology and toxicology, inability to administer medicines, and difficulty in achieving injection administration, so as to reduce vascular irritation and potential toxicity, and facilitate clinical use The effect of using
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: The monoglucoside injection of the cycloastragenol of 2.0mg / ml without solubilizing agent Prescription Screening
[0043] When the monoglucoside concentration of table 1 cycloastragenol is 2.0mg / ml, the non-aqueous solvent ratio screening table without solubilizer
[0044]
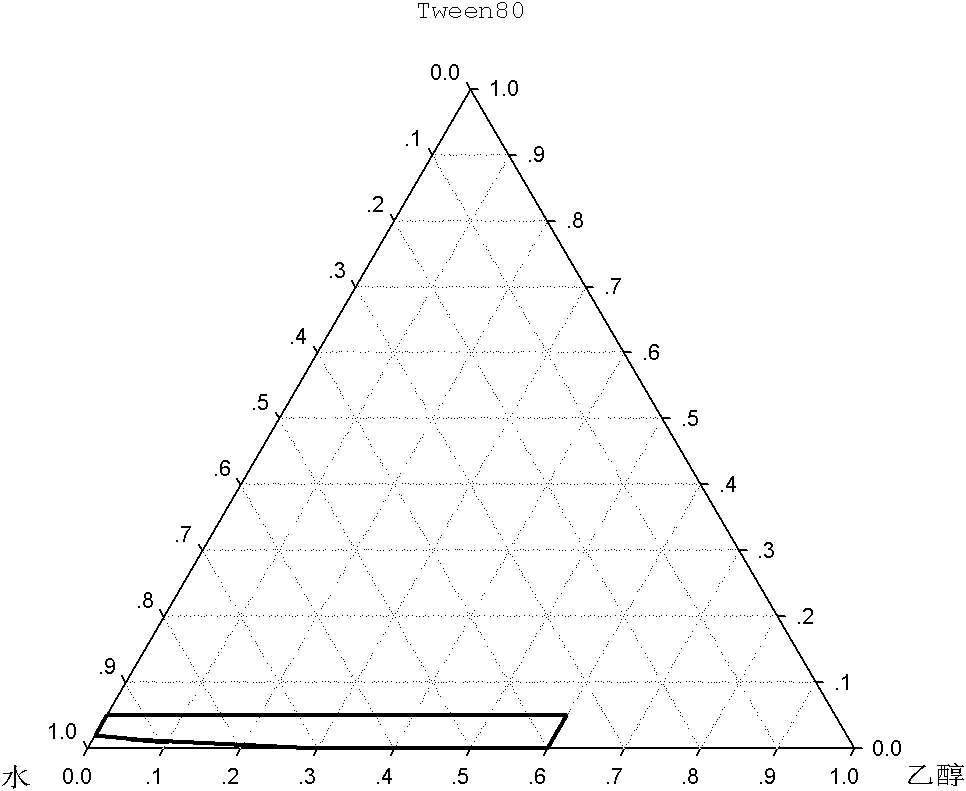
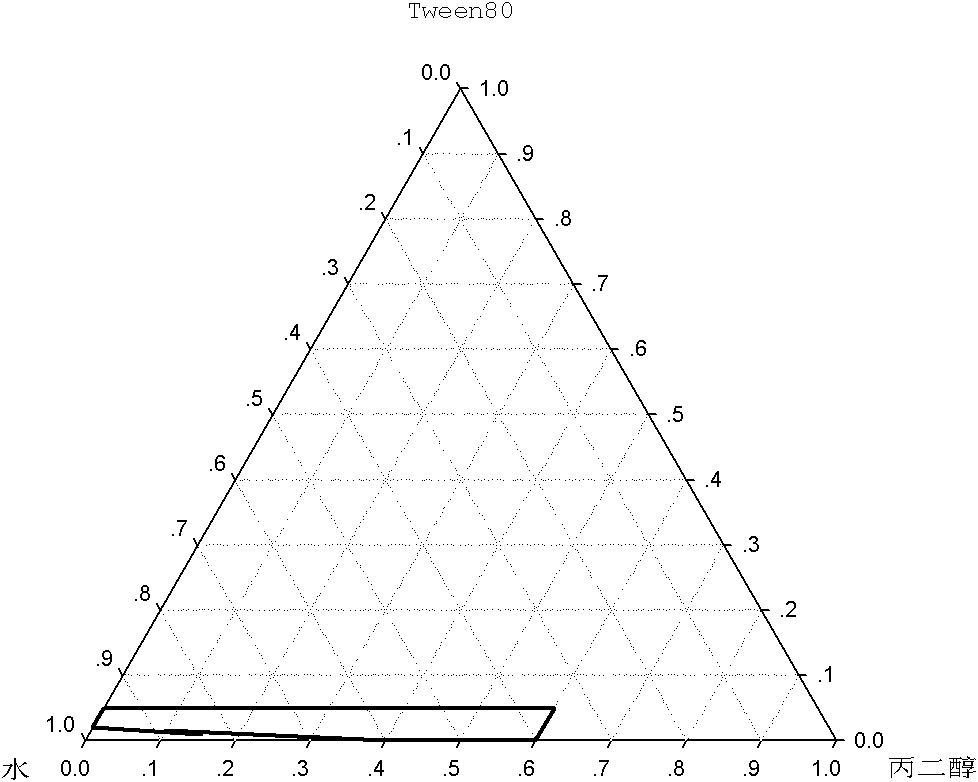
[0045] A 2.0 mg / ml cycloastragenol monoglucoside solution was prepared according to the ratio of non-aqueous solvents in Table 1, and the physical stability under refrigeration was tested. The results show that when ethanol and PEG400 are selected as the non-aqueous solvent at the same time, the physical stability of the liquid medicine under refrigerated conditions can be guaranteed only when the amount of ethanol and PEG 400 are also 25%. During the solvent, the physical stability of the medicinal solution under refrigerated conditions can be guaranteed only when the ethanol consumption is 20%, and the propylene glycol consumption is also 20%.
Embodiment 2
[0046] Embodiment 2: Treatment of monoglucoside injection of cycloastragenol containing 2.0 mg / ml of solubilizing agent party screening
[0047] When the monoglucoside concentration of table 2 cycloastragenol is 2.0mg / ml, the non-aqueous solvent containing solubilizing agent and the ratio screening table of solubilizing agent
[0048]
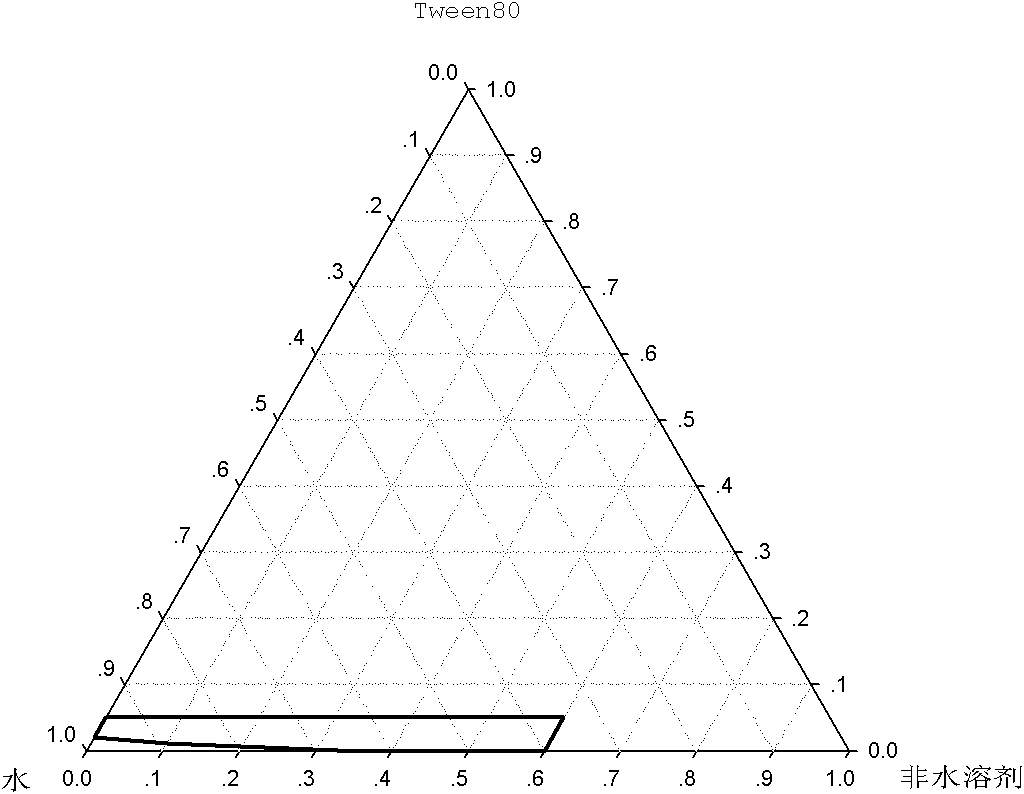
[0049] According to the ratio of non-aqueous solvent and solubilizer in Table 2, a 2.0 mg / ml cycloastragenol monoglucoside solution was prepared, and the physical stability test under refrigerated conditions was carried out. The results show that when ethanol and Tween80 are selected, the amount of ethanol is 5%, the amount of Tween80 is 1.5%, or the amount of ethanol is 10%, and the amount of Tween80 is 1.0%, which can ensure the physical stability of the liquid medicine under refrigerated conditions; When propylene glycol and Tween80 are used, the consumption of propylene glycol is 10%, and the consumption of Tween80 is 1.5%, or the co...
Embodiment 3
[0051] Embodiment 3: the monoglucoside injection of the cycloastragenol of 5.0mg / ml without solubilizing agent Prescription Screening
[0052] When the monoglucoside concentration of table 3 cycloastragenol is 5.0mg / ml, the non-aqueous solvent ratio screening table without solubilizer
[0053]
[0054] According to the ratio of non-aqueous solvents in Table 3, a 5.0 mg / ml cycloastragenol monoglucoside solution was prepared, and the physical stability test under refrigerated conditions was carried out. The results show that when the amount of ethanol is 25%, and the amount of propylene glycol is also 25%, the physical stability of the medicinal solution under refrigerated conditions can be guaranteed.
[0055] Prepare a 5.0 mg / ml cycloastragenol monoglucoside solution according to the ratio of 25% ethanol and 25% propylene glycol. When diluted with 5% glucose injection, when the dilution factor is below 5 times, crystals will precipitate within 10 minutes ; When the dil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com