Reductive composition for preparing 24-epibrassinolide
A kind of technology of epibrassinolide and composition, applied in the field of reducing composition for preparing 24-epibrassinolide, can solve the problems of non-reaction, low product yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] A novel oxidation-free low-temperature preparation method of 24-epibrassinolide, comprising the following steps:
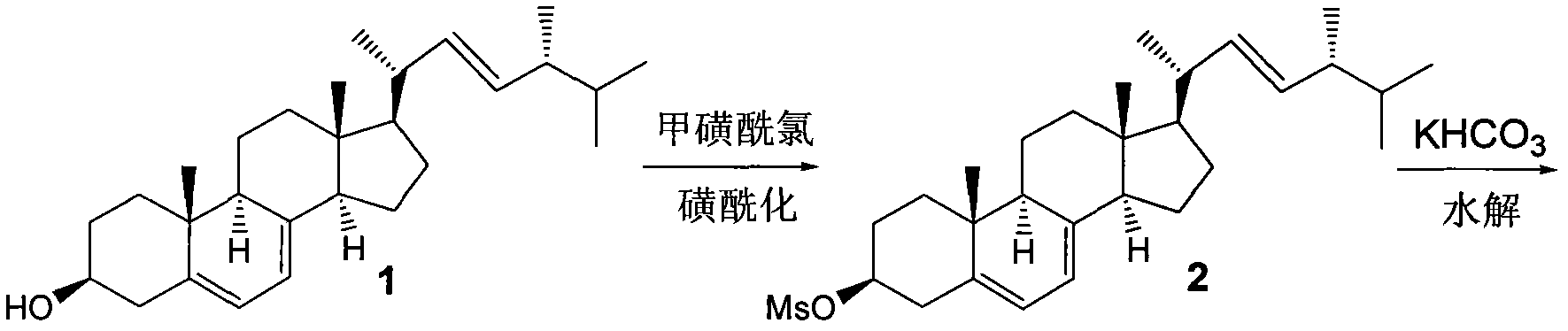
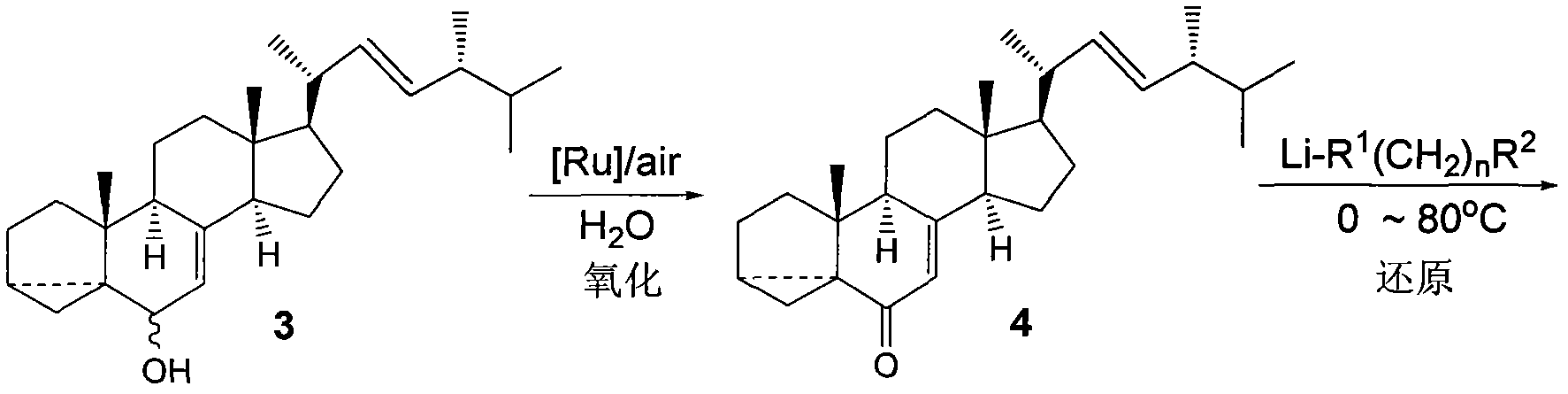
[0025] According to the existing synthesis process, starting from raw material 1, it is prepared in three steps Figure 5 The first compound 3 shown.
[0026] b. The first compound 3 is oxidized with [Ru]-air to Image 6 The second compound shown in 4
[0027] c. The second compound 4 and Li-R 1 (CH 2 ) n R 2 The reducing system of the composition reacts at 0-80°C
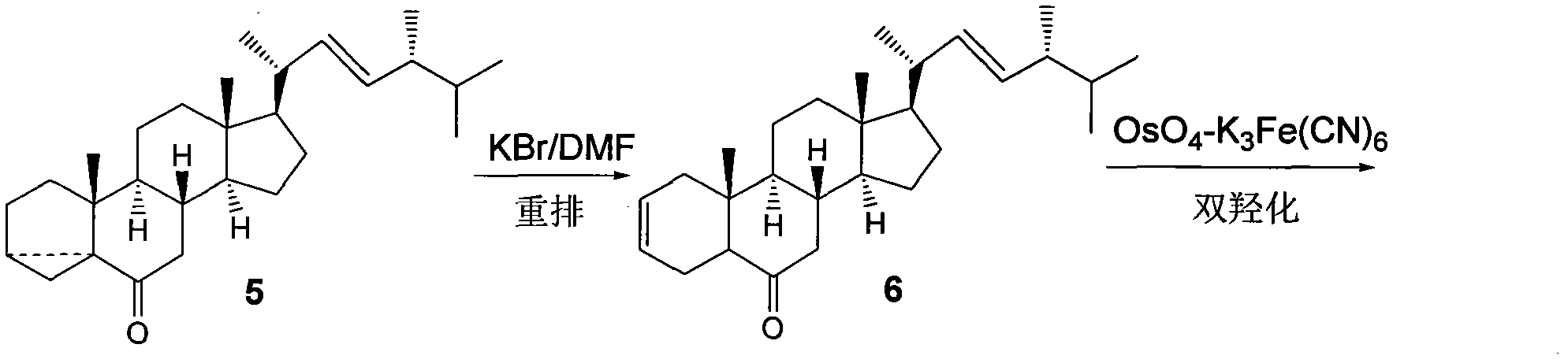
[0028] transform into Figure 7 The third compound 5 shown.
[0029] d. The third compound 5 is prepared by rearrangement, dihydroxylation and lactonization according to the existing synthesis process Figure 8 The final product 24-epibrassinolide 8 is shown.
[0030] The synthesis of the first compound (also called the first intermediate) 3 into the second compound (also called the second intermediate) 4 was carried out using a novel recyclable ruthenium catalyst in water under air ox...
Embodiment 1
[0054] Sulfonylation of ergosterol:
[0055] like figure 1 As shown, dissolve ergosterol (200g, 0.55mol) in 1.0 liter of anhydrous pyridine, cool down to -5~10°C, slowly add a solution of methanesulfonyl chloride in pyridine (100g, 0.55mol. Dissolve in 200mL of anhydrous in pyridine). After the reaction is completed, the reaction solution is poured into a vigorously stirred ice-salt water bath, and a large amount of solids are precipitated. After standing for a period of time, the supernatant is poured out, and then rinsed once with ice-salt water. After standing, the supernatant is poured out, and then the The material was suction filtered, washed with water, and dried to obtain 290 g of sulfonylated ergosterol, with a yield of 104%.
[0056] 1 H NMR (400M Hz, CDCl 3 )δ0.63(3H, s, 18-H), 0.82(3H, d, J=6.9Hz, 26-H), 0.84(3H, d, J=6.9Hz, 27-H), 0.92(3H, d, J=6.9Hz, 28-H), 0.96(3H, s, 19-H), 1.04(3H, d, J=6.9Hz, 21-H), 1.22-2.15(20H, m), 3.02( 3H, s, SO 2 CH 3 ), 4.63(1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com