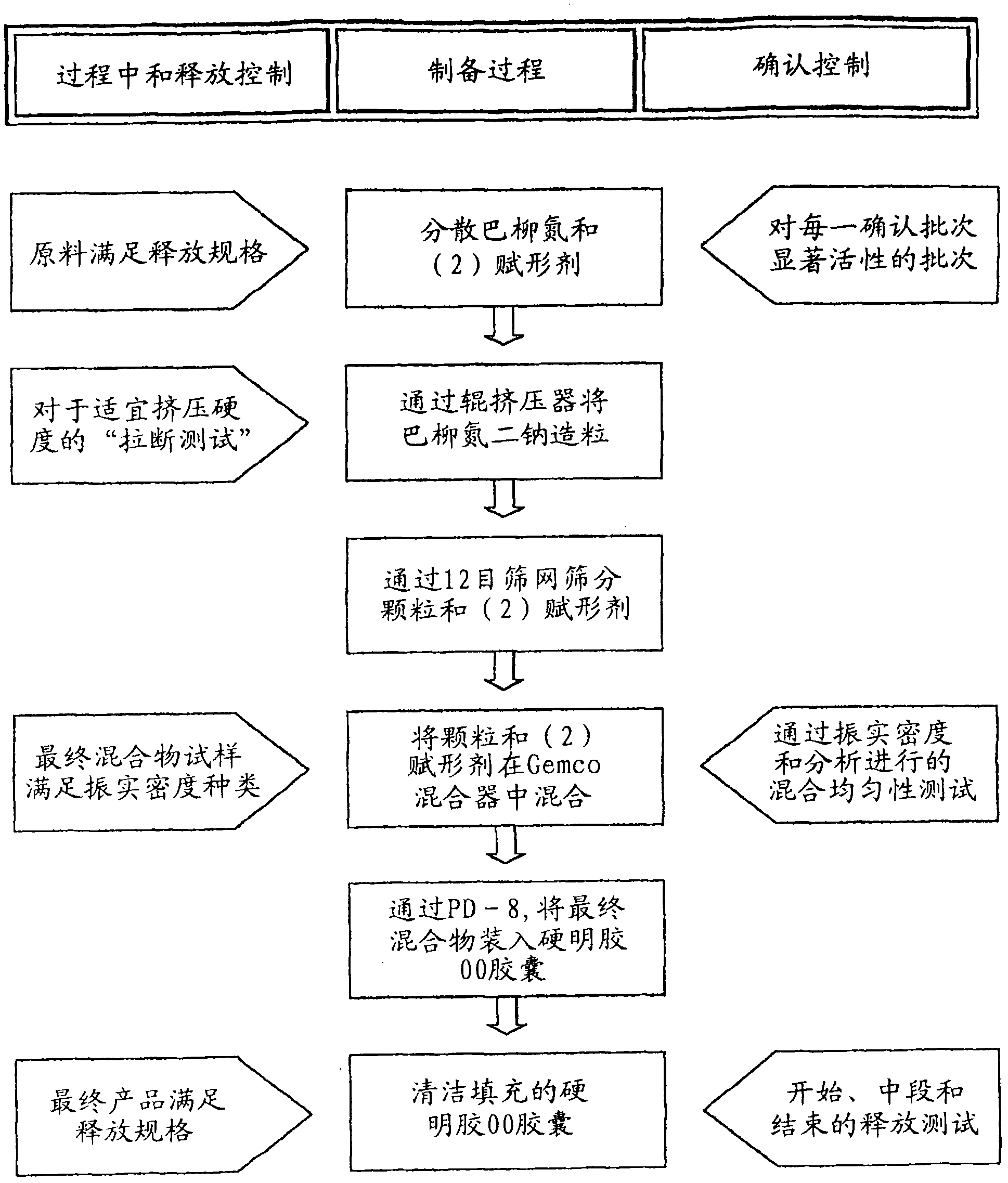
Balsalazide formulations and manufacture and use thereof
A technology of balsalazide and balsalazide disodium, which is applied in the directions of pill delivery, medical preparations without active ingredients, and medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0103] The preparation method of balsalazide tablet
[0104] Shown below is an exemplary method of preparing balsalazide tablets:
[0105] initial weighing
[0106] An appropriate amount of balsalazide disodium and other suitable excipients such as hypromellose and magnesium stearate are dispensed.
[0107] wet granulation
[0108] The balsalazide disodium hypromellose was granulated using a low shear (satellite) mixer. The wet granules were tray dried in an oven to a moisture level of about NMT 2.0%.
[0109] to grind
[0110] The dried granules are milled through a Fitzpatrick mill equipped with, for example, a #2AA mesh stainless steel screen with knives advancing at medium speed.
[0111] final mix
[0112] After milling, the compacted granules were charged to a V-blender and blended with magnesium stearate.
[0113] compression
[0114] The final blend was compressed into 1100 mg tablets using an automatic tablet press.
[0115] tablet coating
[0116] The compr...
Embodiment 1
[0153] Colazal Capsules, 750mg Dissolution Test
[0154] Material
[0155] High-purity water (18MΩ resistivity or higher)
[0156] Balsalazide disodium reference standard
[0157] Monobasic Sodium Phosphate, ACS Reagent Grade
[0158] 10N NaOH
[0159] Type 316 Stainless Steel Coil Hood, Laboratory Quality Accessory, Catalog Number: CAPWHT-4S
[0160] Gelman nylon filter, 25mm, 0.2μm pore size, Part No. 4436T
[0161] Vankel 10-µm full-flow filter, part number 17-400
[0162] Specifications (refer to current USP chapter )
[0163] Phase 1 After 30 minutes, each formulation unit must be not less than 75% dissolved (Q=70%)
[0164] Stage 2 After 30 minutes, the average of the 12 formulation units from Stage 1 and Stage 2 must be not less than 70% dissolved. After 30 minutes, each formulation unit must be not less than 55% dissolved.
[0165] Stage 3 After 30 minutes, the average of the 24 formulation units from stages 1, 2 and 3 must be not less than 70% dissolved. Aft...
Embodiment 2
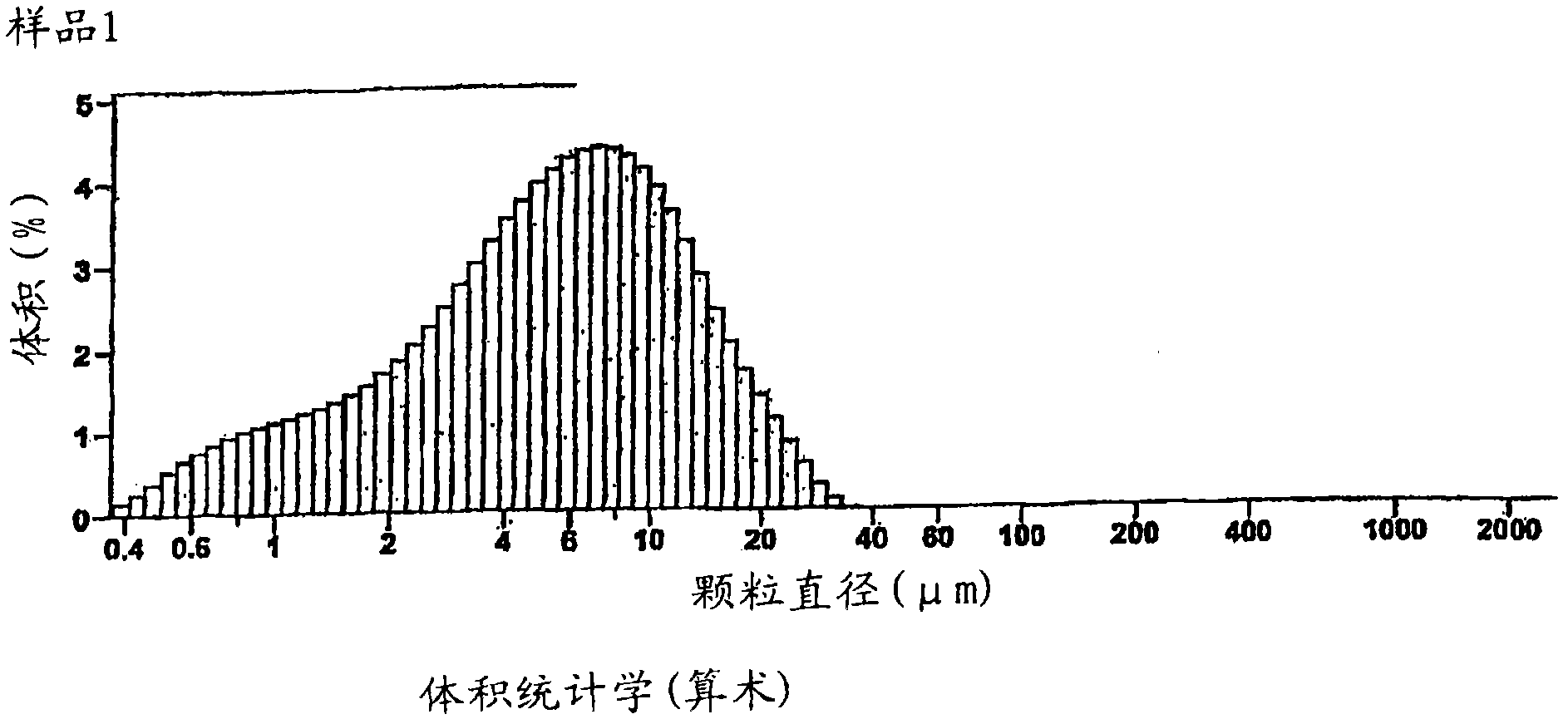
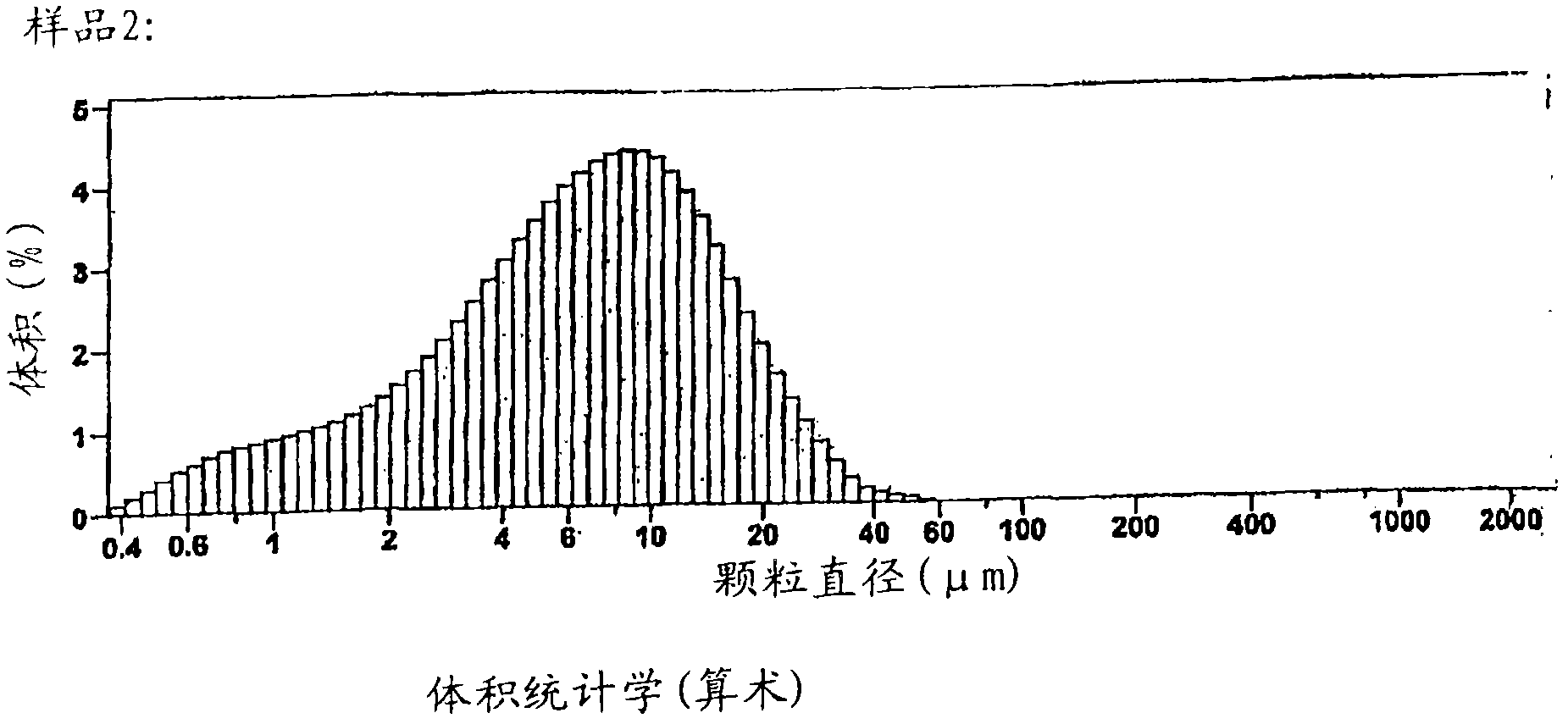
[0209] For the arithmetic statistics of sample 1, figure 2 The data, arithmetic statistics characterizing the surface area, particle size and cumulative volume of a batch of drug (feed material) are shown and tabulated. The particle size distribution of this batch is in figure 2 shown in .
[0210] Sample 1:
[0211] Optical model: Fraunhofer.rfz
[0212] LS 200 small volume module
[0213] Calculation from 0.375μm to 2000μm
[0214] Volume: 100%
[0215] Average: 6.967μm
[0216] Median value: 5.584 μm
[0217] Specific surface area: 19389cm 2 / mL
[0218] %<10 25 50 75 100
[0219] μm 1.266 2.827 5.584 9.624 43.67
[0220] table 5
[0221]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com