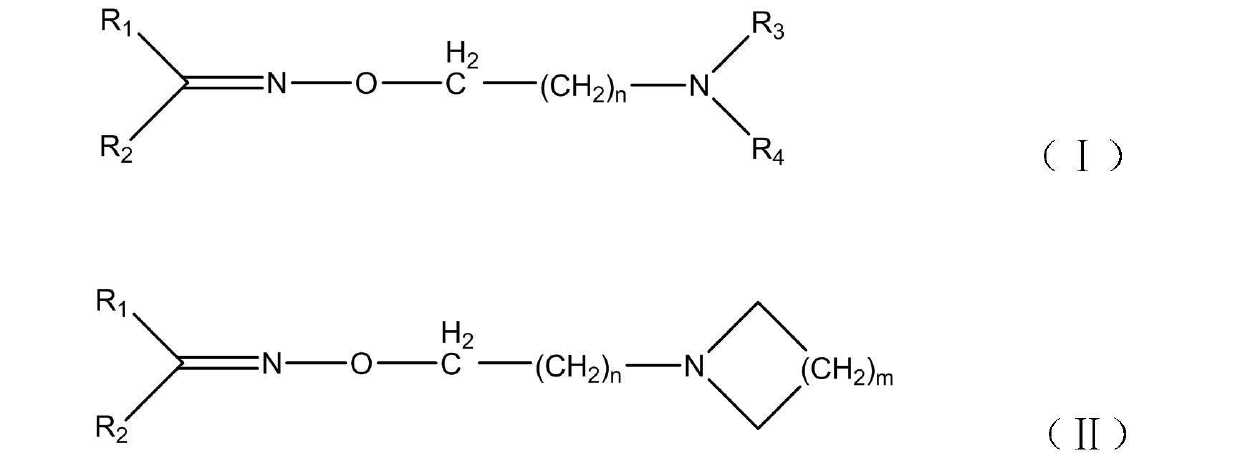
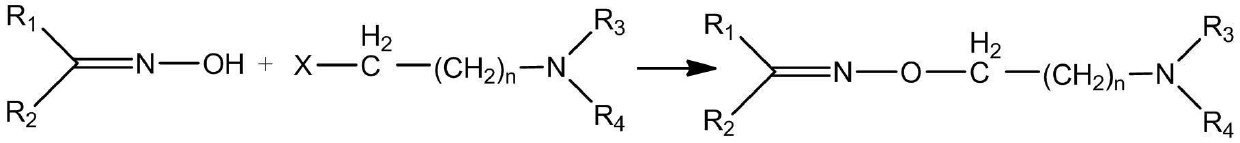
Oxime ether amine compound or oxime ether amine salt, composition containing oxime ether amine compound or oxime ether amine salt and application thereof
A technology of amine compounds and compositions, which is applied in the application of plant rooting accelerators, oxime ether amine compounds or their salts, and the field of compositions containing oxime ether amine compounds or their salts, which can solve the problem of high price and poor water solubility , damage to plants and other issues, to achieve the effects of a wide range of concentration, promotion of average root length or rooting height, and strong average root length or rooting height
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Synthesis of N,N-piperidinyl-2-(benzaldoximeoxy)ethylamine (compound 1c)
[0020] Add 6.7 g (0.05 mol) of benzaldehyde oxime, 2.4 g (0.06 mol) of sodium hydroxide and 15.0 mL of water into a dry 100 mL two-necked flask. Adjust the temperature to 50°C and stir to dissolve for 30 min, then add dropwise a solution of 11.0 g (0.06 mol) N-(2-chloroethyl)piperidine hydrochloride and 15.0 mL of water for 30 min. After the dropwise addition, adjust the temperature to 100 °C and continue the reaction for 8 h. At this time, the reaction solution clearly separated, cooled, and the organic phase was separated. The aqueous layer was extracted with anhydrous ether (3×20.0 mL), and the organic phases were combined, washed with water, and dried. , the solvent was evaporated under normal pressure to obtain a dark brown liquid, which was passed through a silica gel column with mobile phases of ethyl acetate and methanol to obtain the target product with a yield of 48.2%. The ...
Embodiment 2
[0022] Example 2: Synthesis of N,N-dimethyl-2-(4-chlorobenzaldoximeoxy)ethylamine (compound 2a)
[0023] Colorless oily liquid, IR (KBr) υ, cm -1 : IR (KBr) υ, cm -1 : 3028(Ar-H), 1648(C=N), 1600, 1492, 1469 (skeleton vibration of benzene ring), 1038 (C-O). 1 H NMR (600MHz, CDCl 3 ) δ: 2.322(s, 6H), 2.662-2.679(t, 2H, J = 6.0 Hz), 4.273-4.291(t, 2H, J = 6.0 Hz), 7.326-7.343(dd, 2H, J = 6.0 Hz , J = 2.4 Hz), 7.498-7.516(dd, 2H, J = 6.0 Hz, J = 2.4 Hz), 8.076-8.080(d, 1H, J = 2.4 Hz)
Embodiment 3
[0024] Example 3: Synthesis of N,N-diethyl-2-(4-chlorobenzaldoximeoxy)ethylamine (compound 2b)
[0025] Colorless oily liquid, IR (KBr) υ, cm -1 : IR (KBr) υ, cm -1 : 3034(Ar-H), 1651(C=N), 1595, 1465 (skeleton vibration of benzene ring), 1043 (C-O). 1 H NMR (600MHz, CDCl 3 ) δ: 1.049-1.069(t, 6H, J = 6.0 Hz), 2.604-2.641(q, 4H, J = 7.2 Hz), 2.789-2.817(t, 2H, J = 6.0 Hz), 4.247-4.268(t , 2H, J = 6.0 Hz), 7.328-7.342(d, 2H, J = 8.4 Hz), 7.502-7.516(d, 2H, J = 8.4 Hz), 8.053(s, 1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com