Synthetic method of glycyrrhetinic acid ester derivative
A technology of glycyrrhetinic acid ester and glycyrrhizic acid, which is applied in the field of synthesis of glycyrrhetinic acid ester derivatives, can solve the problems of high equipment requirements, long reaction time, and unsuitability for industrial production, and achieve high drug efficacy and reduce waste of resources Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
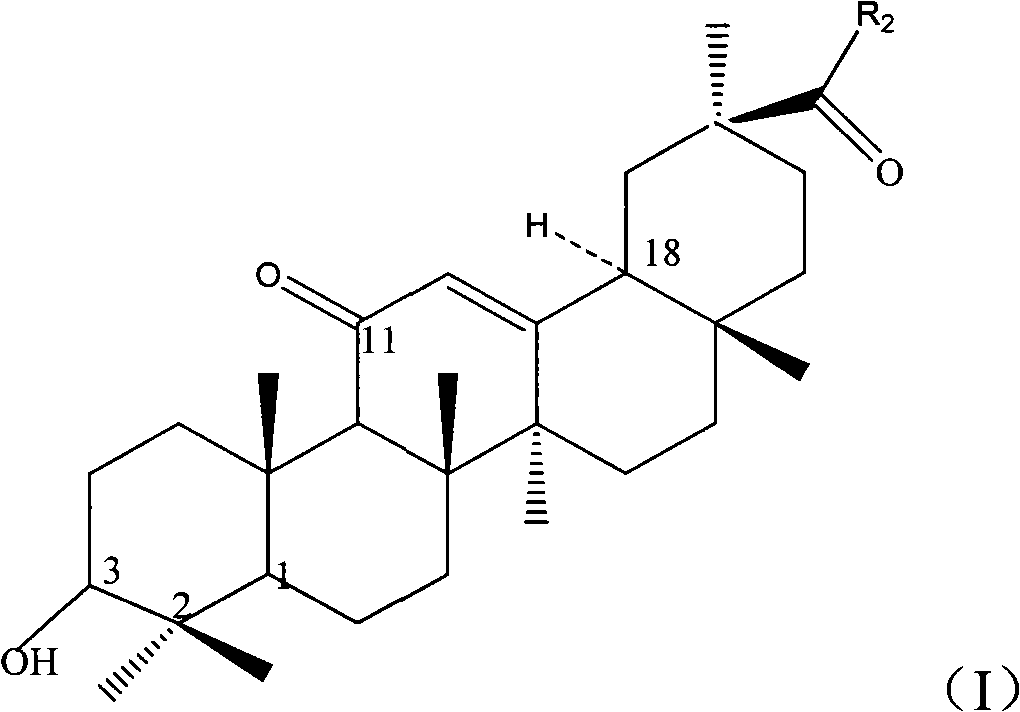
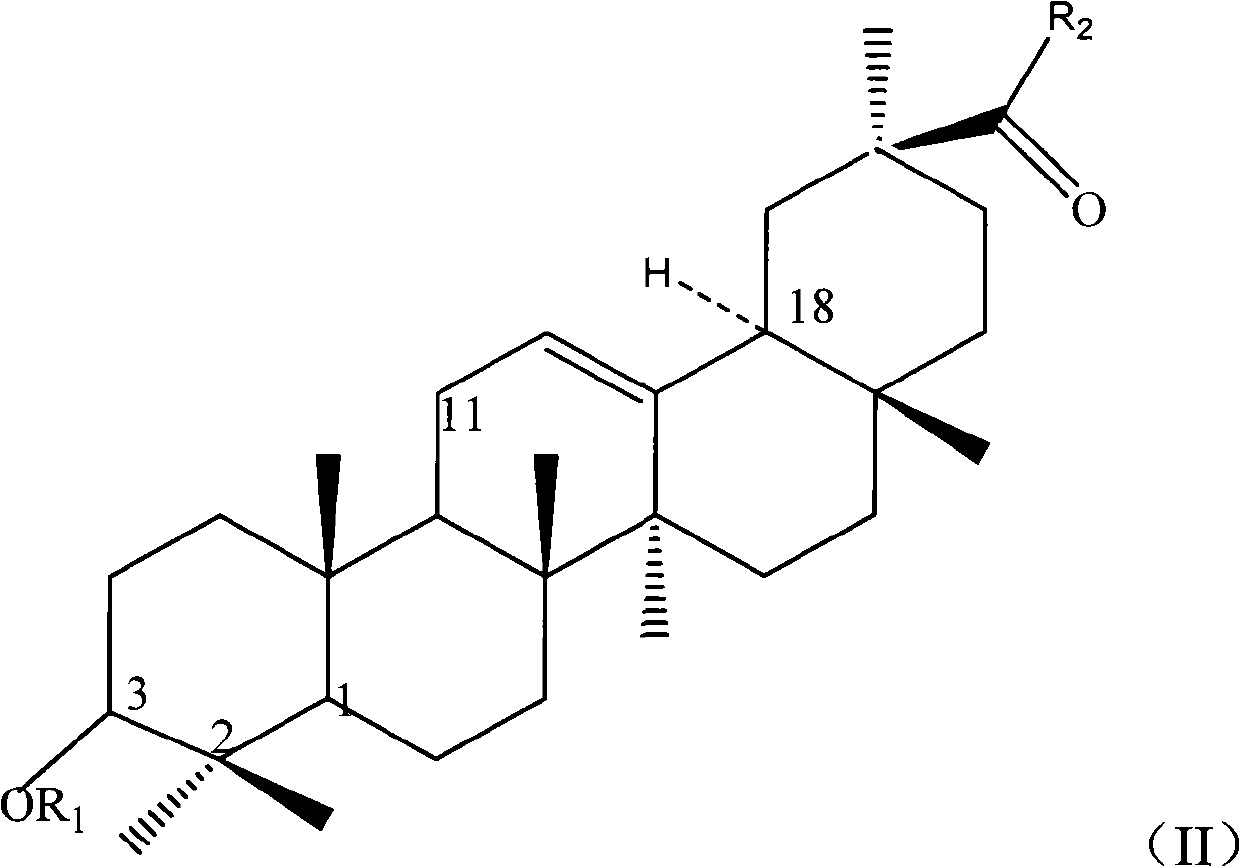
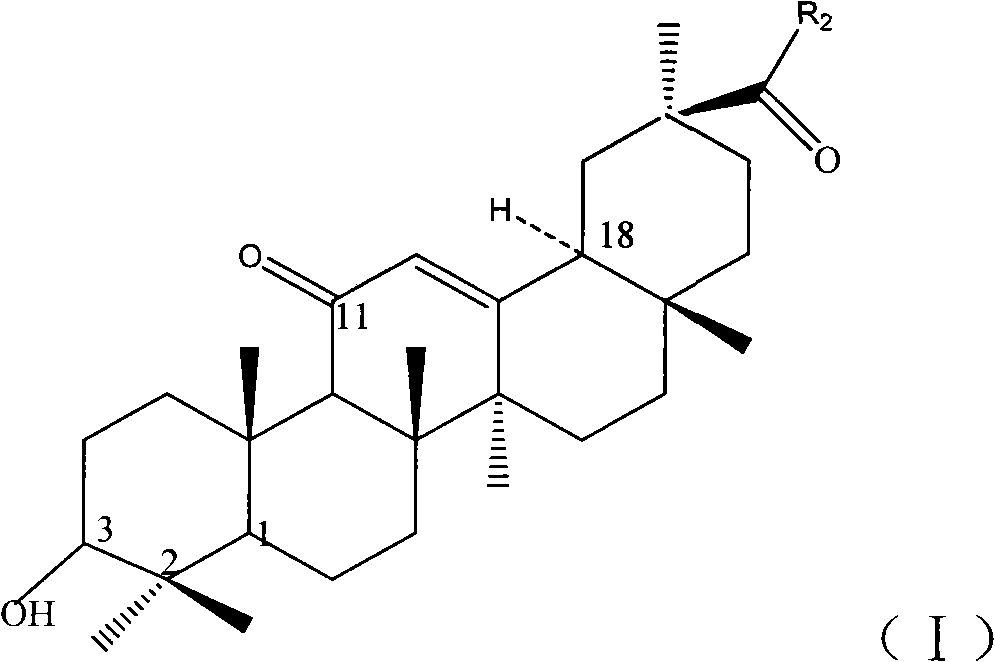
Image
Examples
Embodiment 118
[0043] The synthesis of embodiment 118β-methyl glycyrrhetinate
[0044] Method 1. Add 10 g of 18β-glycyrrhizic acid to 100 ml of anhydrous methanol, add 5 ml of acetyl chloride, heat to reflux for 2 hours, add 100 ml of water, cool, crystallize a solid, filter, refine with ethanol / water, and dry to obtain the title compound.
[0045] Method 2. Add 20g of 18β-glycyrrhizic acid monoammonium salt into 100ml of anhydrous methanol, add 10ml of acetyl chloride, heat and reflux for 2 hours, the color turns brown, add 200ml of water, cool, crystallize a solid, filter, and refine with ethanol / water , dried to obtain the title compound in a yield of 79%.
[0046] IR: v as (-OH)3387cm -1 , v as (-COOCH 3 )1725cm -1 , v as (=O)1657, 1621cm -1 , v as (A district) 1387, 1361cm -1 , v as (Area B) 1322, 1278, 1246cm -1 .
Embodiment 218
[0047] The synthesis of embodiment 218α-ethyl glycyrrhetinate
[0048] Method 1. Add 10 g of 18α-glycyrrhizic acid into 100 ml of absolute ethanol, add 5 ml of acetyl chloride, heat to reflux for 2 hours, add 100 ml of water, cool, crystallize a solid, filter, refine with 80% ethanol, and dry to obtain the title compound. Yield 85%.
[0049] 1 H-NMR: 0.72(s, 3H), 0.81(s, 3H), 1.00(s, 3H), 1.14(s, 3H), 1.20(s, 3H), 1.22(s, 3H), 1.26(t, 3H), 1.35(s, 3H), 4.14(q, 2H), 5.57(s, 1H)
[0050] 13 C-NMR (ppm): 14.13, 15.62, 15.94, 16.47, 17.54, 18.49, 20.65, 20.75, 26.65, 27.22, 28.07, 28.40, 31.70, 33.75, 35.45, 35.97, 36.84, 37.60, 39.0792, 40.39. , 43.80, 44.89, 54.99, 60.42, 60.66, 78.70, 124.08, 165.64, 178.20, 199.74
[0051] Method 2. Add 10g of 18α-glycyrrhizic acid into 100ml of absolute ethanol, add 1ml of concentrated sulfuric acid, heat and reflux for 8 hours, add 100ml of water, cool, crystallize a solid, filter, refine with ethanol / water, and dry to obtain the title ...
Embodiment 7
[0076] Embodiment 7 Anti-inflammatory effect of the compound of the present invention
[0077] The anti-inflammatory effect of the drug was observed by injecting carrageenan into the paws of rats to induce swelling.
[0078] in:
[0079] (1) Experimental materials
[0080] Animals: male SD rats, 150-180g;
[0081] Inflammator: carrageenan;
[0082] Test substance: use 1% CMC-Na to make 11-deoxy-18α ethyl glycyrrhetinate to the required concentration;
[0083] Positive drug: indomethacin, prepared to the required concentration with 1% CMC-Na;
[0084] (2) Experimental method
[0085] 50 rats were randomly divided into 5 groups with 10 rats in each group, which were model group, positive group (administered indomethacin 10 mg / kg), and test drug dosage groups (30, 60, 120 mg / kg). Animals in each group were administered continuously for 3 days, and before the last administration, the volume of the left hind paw of the rats before administration was measured by micropipette mea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com