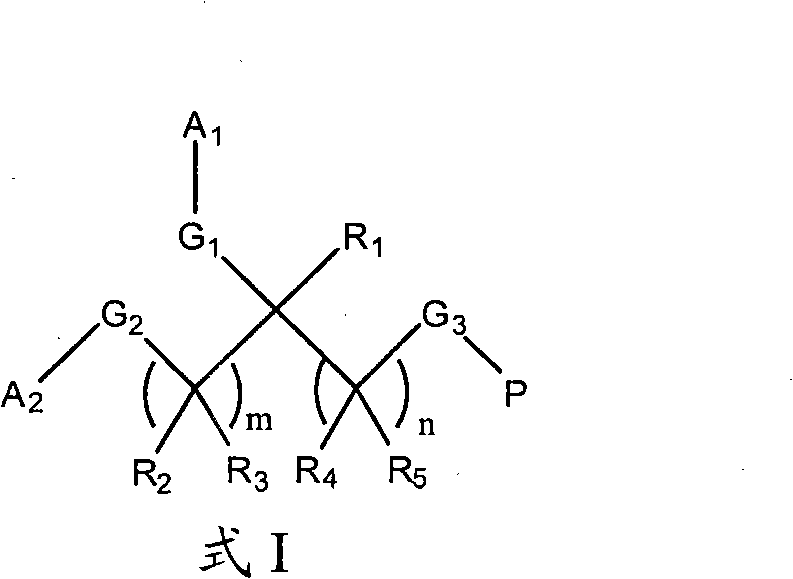
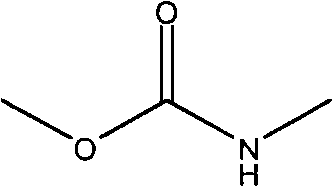
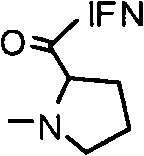
Therapeutic uses of protein-polymer conjugates
A technology of conjugates and uses, applied in the direction of peptide/protein components, animal/human proteins, active ingredients of heterocyclic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0062] Preparation of di-PEG aldehyde
[0063]
[0064] 20kD PEGO(C=O)OSu was prepared from 20kD mPEGOH purchased from (SunBio Inc., CA, USA) according to the method described in Bioconjugate Chem. 1993, 4, 568-569.
[0065] A solution of 6-(1,3-dioxolan-2-yl)hexane-1,5-diamine in dichloromethane (11.97 g solution containing 9.03 mg diamine, 47.8 μmol) was added to a solution containing 20 kD PEGO (C=O)OSu (1.72 g, 86.0 μmol) in a flask. After complete dissolution of PEGO(C=O)OSu, N,N-diisopropylethylamine (79 μL, 478 μmol) was added. The reaction mixture was stirred at room temperature for 24 hours, then methyl tert-butyl ether (200 mL) was added dropwise with stirring. The resulting precipitate was collected and dried under vacuum to yield the bis-PEG acetal (1.69 g, 98%) as a white solid.
[0066] 1 H NMR (400MHz, d 6 -DMSO) δ7.16(t, J=5.2Hz, 1H), 7.06(d, J=8.8Hz, 1H), 4.76(t, J=4.8Hz, 1H), 4.10-3.95(m, 4H), 1.80-1.65 (m, 1H), 1.65-1.50 (m, 1H), 1.48-1.10 (m, 6H)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com