Preparation of panaxadiol saponins component and pharmaceutical application for prevention and treatment of Parkinson disease
A technology of ginseng diol saponins and Parkinson's disease, applied in the field of medicine, can solve problems such as difficult medicinal value, involuntary movement, abnormality, etc., and achieve the effect of maintaining or restoring self-care ability and quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Preparation of ginsengdiol saponin component-A (PDSF-A) from ginseng rhizome medicinal materials
[0059] 1. Separation and purification of ginsengdiol saponins component-A (PDSF-A) :
[0060] Ginseng rhizome medicinal material (8000g, sample-A, the five ginsengdiol saponins Rb contained in it 1 , Rc, Rb 2 , Rb 3 The content of Rd and Rd and their total content are shown in Table 5) After being crushed into coarse powder, the extract was percolated and extracted with 50% ethanol aqueous solution until the extract was colorless. The percolate was combined to recover the ethanol under reduced pressure and freeze-dried to obtain the extract (678.6 G). The extract was dissolved in 3000 ml of 45% ethanol aqueous solution, and the sample solution was separated by macroporous adsorption resin (Diaion HP-20, 6000 g) column chromatography. Firstly, it was eluted with 45% ethanol aqueous solution until the eluent had no obvious saponin The reaction was eluted with 70% et...
Embodiment 2
[0083] Example 2 Preparation of ginsenoside component-B (PDSF-B) from American ginseng rhizome medicinal materials
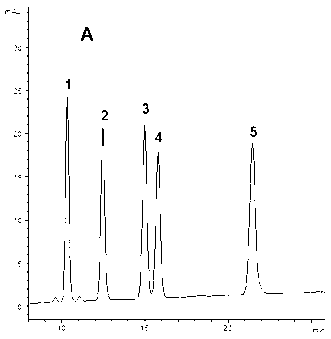
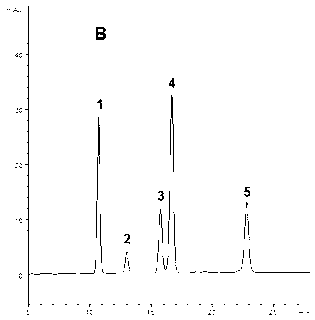
[0084] Use American ginseng root and stem medicinal materials (7000 grams, sample-B, which contains five ginsengdiol saponins Rb 1 , Rc, Rb 2 , Rb 3 The content of and Rd and their total content are shown in Table 5, and the HPLC spectrum is shown in Figure 1-3 ), using the same method as in Example 1 to prepare the ginsenoside component - B (PDSF-B, 101.3 g). HPLC analysis shows: PDSF-B also contains ginsenoside Rb 1 , Rc, Rb 2 , Rb 3 And Rd five kinds of ginsenosides, the total content of these five kinds of saponins is 91.60%, of which ginsenoside Rb 1 The highest content, followed by Rb 3 , Rd, Rb 2 And Rc; and Rb in ginsengdiol saponin component-B 1 / Rc / Rb 2 / Rb 3 / Rd content ratio and Rb in American ginseng root and stem medicinal materials (Sample-B) 1 / Rc / Rb 2 / Rb 3 The ratio of / Rd content is basically the same (Table 5).
Embodiment 3
[0085] Example 3 Preparation of ginsengdiol saponins component -C ( PDSF -C)
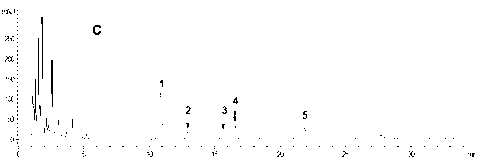
[0086] Purchased commercial ginseng rhizome total saponins (sample-C 1 , Its total saponins content ≥80%) and American ginseng stems and leaves total saponins (sample-C 2 , Its total saponins content ≥80%) HPLC analysis shows that the five ginsengdiol saponins contained in these two ginseng total saponins products have their own characteristics, namely: Sample-C 1 Both contain ginsenoside Rb 1 , Rc, Rb 2 , Rb 3 And Rd, but Rb 3 The content is very low (see attached diagram 2-1 And Table 5); Sample-C 2 Mainly containing Rb 3 And Rd, and Rb 1 , Rc and Rb 2 The content is very low (see attached Figure 2-2 And Table 5). This implementation comprehensively utilizes these two samples (Sample-C 1 And sample-C 2 ) Is used as the raw material to prepare the active component of ginsengdiol saponins of the present invention, especially the total content of five ginsengdiol saponins is ≥90%, and the content of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com