Catalyst for toluene chlorination and preparation method thereof
A catalyst and main catalyst technology, applied in the field of chemistry, can solve the problems of restricting industrial application, high production cost, destroying the lattice of zeolite molecular sieve and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
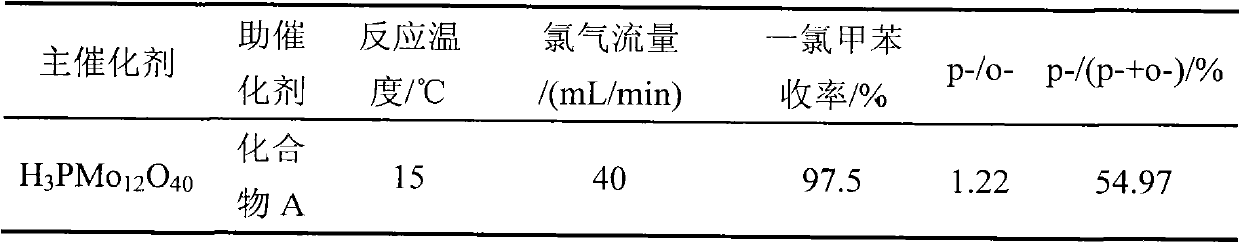
[0024] 1.8gNa 2 HPO 4 12H 2 O and 14.5gNa 2 MoO 4 2H 2 O was dissolved in 30 mL and 100 mL of distilled water respectively, and the two solutions were mixed and added into a 250 mL three-neck flask, heated to boiling, refluxed for 1 hour, stopped heating, and added 30% hydrochloric acid while stirring until the pH of the solution = 2.0, And continue to stir to room temperature, add 80mL ether to the reaction solution, shake fully, then add 30% hydrochloric acid and continue shaking until the solution is divided into three layers after standing, and the yellow oily substance in the lower layer is heteropolyacid ether compound. Take out the ether compound in the lower layer, blow off the ether, add 2 mL of distilled water, put it in a vacuum desiccator until the crystals are completely precipitated, filter and dry to obtain 12-molybdophosphoric acid (H 3 PMo 12 o 40 ) crystal 7.8g. The resulting 12-molybdophosphoric acid (H 3 PMo 12 o 40 ) is activated in a muffle fur...
Embodiment 2
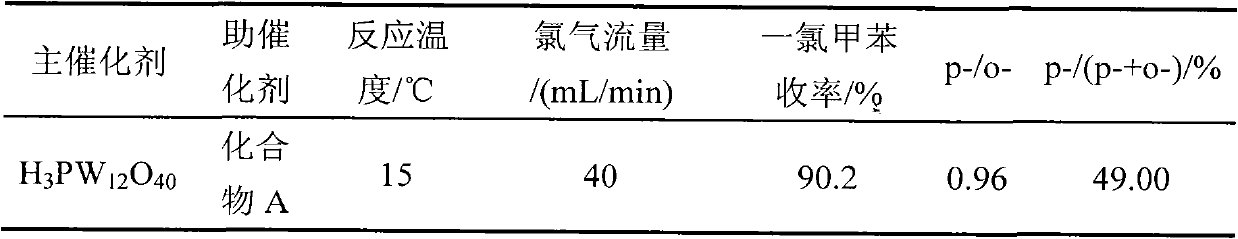
[0030] 1.8gNa 2 HPO 4 12H 2 O and 20.0gNa 2 WO 4 2H 2O was dissolved in 30 mL and 100 mL of distilled water respectively, and the two solutions were mixed and added into a 250 mL three-neck flask, heated to boiling, refluxed for 1 hour, stopped heating, and added 30% hydrochloric acid while stirring until the pH of the solution = 2.0, And continue to stir to room temperature, add 80mL ether to the reaction liquid, shake fully, then add 30% hydrochloric acid and continue shaking until the solution is divided into three layers after standing, the lower white oily substance is heteropoly acid ether compound. Take out the ether compound in the lower layer, blow off the ether, add 2mL of distilled water, put it in a vacuum desiccator until the crystals are completely precipitated, filter and dry to obtain 12-tungstophosphoric acid (H 3 PW 12 o 40 ) crystal 12.9g. The resulting 12-tungstophosphoric acid (H 3 PW 12 o 40 ) is activated in a muffle furnace, the activation te...
Embodiment 3
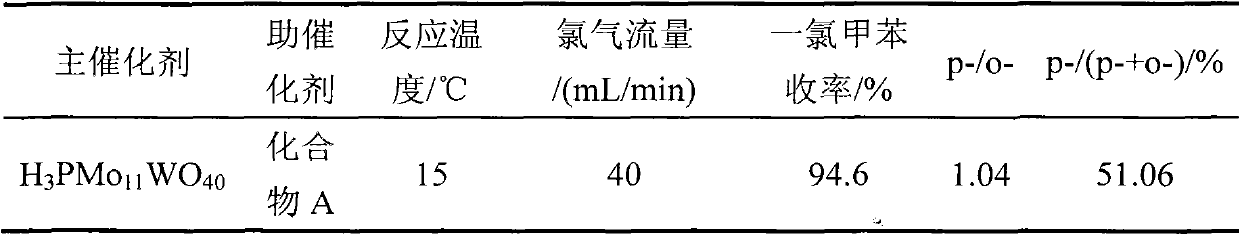
[0036] 1.8gNa 2 HPO 4 12H 2 O and 13.3gNa 2 MoO 4 2H 2 O was dissolved in 30mL and 100mL of distilled water respectively, and the two solutions were mixed and added into a 250mL three-neck flask, heated to boiling, and refluxed for 1h; 1.65gNa 2 WO 4 2H 2 O was dissolved in 15mL of distilled water, and the solution was added to the above reaction solution under stirring conditions, heated to reflux for 6 hours, stop heating, add 30% hydrochloric acid to the solution pH = 2.0 while stirring, continue to stir to room temperature, add Add 80mL of diethyl ether to the reaction solution. After fully shaking, add 30% hydrochloric acid and continue shaking until the solution is divided into three layers after standing still. The yellow oily substance in the lower layer is heteropolyacid ether compound. Take out the ether compound in the lower layer, blow off the ether, add 2 mL of distilled water, put it in a vacuum desiccator until the crystals are completely precipitated, fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com