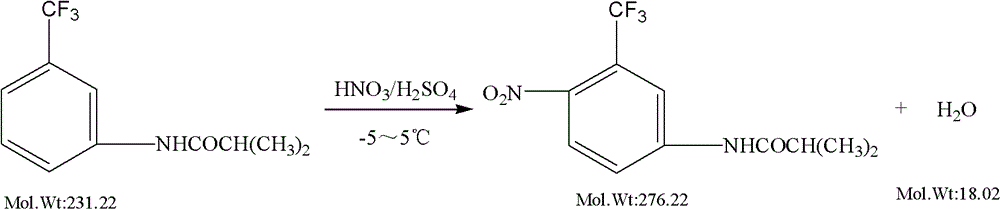
The preparation method of flutamide
A technology of flutamide and isobutyrylaminotrifluorotoluene, applied in the field of medicine, can solve the problems of difficult reaction stirring, long cooling and placing, long production cycle, etc., and achieves the advantages of good product quality, easy temperature control, and shortened production cycle. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 31 g of fuming nitric acid and 248 ml of concentrated sulfuric acid into the reaction pot, start stirring, and cool with ice-salt water. When the temperature dropped to -5°C, 100 g of m-isobutyramide trifluorotoluene was slowly added. After the addition, keep the reaction at 2-5°C for 2 hours. Then, the reaction solution is added dropwise to an ice-salt water system composed of sodium chloride, crushed ice and water for crystallization, and the dropping temperature is controlled at -5 to 5°C. The dropwise addition time is about 1.5 hours, the solid matter is precipitated, filtered, and then soaked with drinking water, the filter cake, 500ml toluene, and 1g activated carbon are added to the decolorization pot, start stirring, and heat the temperature to 80-85°C. Heat to reflux for 0.5 hour, filter while hot, cool the filtrate below 0°C to crystallize, and centrifuge to obtain the wet crude product of flutamide, dry the wet crude product below 80°C to obtain 59.5 g o...
Embodiment 2
[0025] Add 29 g of fuming nitric acid and 250 ml of concentrated sulfuric acid into the reaction pot, start stirring, and cool with ice-salt water. When the temperature dropped to -5°C, 100 g of m-isobutyramide trifluorotoluene was slowly added. After the addition, keep the reaction at 1-5°C for 2 hours. Then, the reaction solution is added dropwise to an ice-salt water system composed of calcium chloride, crushed ice and water for crystallization, and the dropping temperature is controlled at -5 to 0°C. The dropwise addition time is about 1 hour, the solid matter is precipitated, filtered, and then soaked with drinking water, the filter cake and 500ml toluene are added to the crystallization pot, the stirring is started, and the temperature is heated to 75-80°C. After heating and dissolving, the filtrate was crystallized by cooling below 0°C, and centrifuged to obtain a wet crude product of flutamide, which was dried below 80°C to obtain 60.5 g of a crude product of flutamid...
Embodiment 3
[0027] Add 29 g of fuming nitric acid and 250 ml of concentrated sulfuric acid into the reaction pot, start stirring, and cool with ice-salt water. When the temperature dropped to -5°C, 100 g of m-isobutyramide trifluorotoluene was slowly added. After the addition, keep the reaction at 6-8°C for 1 hour. Then, the reaction solution is added dropwise to an ice-salt water system composed of sodium chloride, crushed ice, and water for crystallization, and the dropping temperature is controlled at -5 to 0°C. The dropwise addition time is about 5 hours, the solid matter is precipitated, filtered, and then soaked with drinking water. Add the filter cake, 500ml toluene, and 1g activated carbon into the decolorization pot, start stirring, and heat the temperature to 80-85°C. Heat to reflux for 0.5 hour, filter while hot, the filtrate crystallizes at 0°C, and centrifuge to obtain the wet crude product of flutamide, dry the wet crude product below 80°C to obtain 56.8 g of the crude prod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com