Method for preparing calcium carbonate powders of different structures and shapes
A technology of calcium carbonate powder and calcium bicarbonate, applied in the direction of calcium carbonate/strontium/barium, nanotechnology, etc., can solve the problems of complex process and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
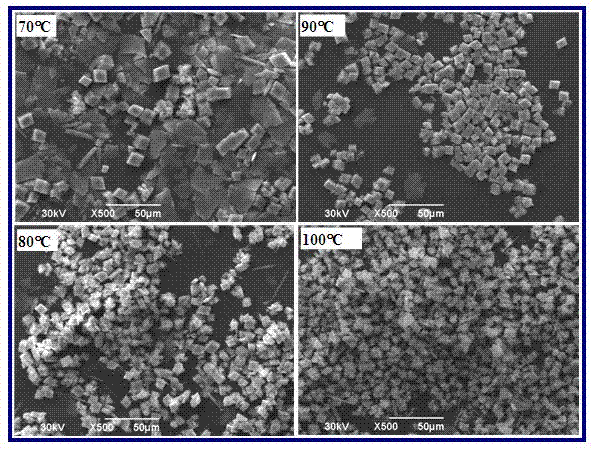
[0033] Add calcium oxide or calcium hydroxide powder into 4 flasks filled with distilled water. After stirring to fully react or dissolve, pass in excess carbon dioxide gas and filter with filter paper to obtain an aqueous solution of calcium bicarbonate; The flask of the calcium aqueous solution was placed in a water bath and heated to 70°C, 80°C, 90°C, and 100°C while stirring and kept for 60 minutes. Calcium bicarbonate is decomposed to form calcium carbonate, which is filtered or centrifuged and dried to obtain calcium carbonate powder. The crystal type and particle morphology of the calcium carbonate powder obtained are as figure 1 , figure 2 Shown. At 70℃, the main crystal phase is rhombohedral calcite, which also contains a large amount of flake vaterite phase and a small amount of rod-shaped aragonite phase; below 80℃, the content of calcite increases, while the amount of vaterite and aragonite All have decreased; below 90℃, the number of calcite further increases, th...
Embodiment 2
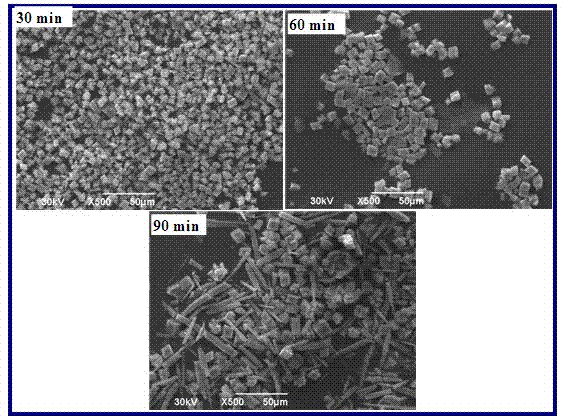
[0035] Add calcium oxide or calcium hydroxide powder to 3 flasks filled with distilled water, after stirring to fully react or dissolve, pass in excess carbon dioxide gas, filter with filter paper to obtain an aqueous solution of calcium bicarbonate; will contain calcium bicarbonate The flask of the aqueous solution was placed in a water bath and heated to 80°C while stirring and kept for 30min, 60min, and 90min respectively. Calcium bicarbonate is decomposed to form calcium carbonate, which is filtered or centrifuged and dried to obtain calcium carbonate powder. The crystal type and particle morphology of the calcium carbonate powder obtained are as image 3 , Figure 4 Shown. The decomposition reaction proceeded to 30 minutes to obtain all rhombohedral calcite phases. When the reaction proceeded to 60 minutes, a small amount of flake vaterite crystal phases appeared, and when the reaction proceeded to 90 minutes, a large number of rod-shaped aragonite phases appeared. .
Embodiment 3
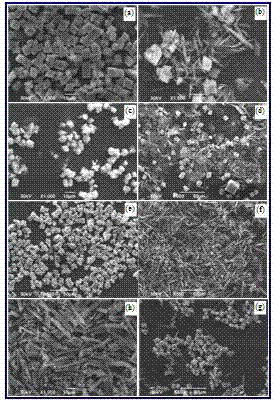
[0037] Add calcium oxide or calcium hydroxide powder to 8 flasks filled with distilled water, after stirring to fully react or dissolve, pass in excess carbon dioxide gas, filter with filter paper to obtain an aqueous solution of calcium bicarbonate; Add different types and quantities of additives to the flask of the aqueous solution (see Table 1), and mechanically stir for 30 minutes to make the additives fully interact with calcium ions. The flask containing the fully mixed calcium bicarbonate aqueous solution is placed in a water bath, heated to 90°C while stirring and kept for 60 minutes. The calcium bicarbonate decomposes to form calcium carbonate, which is filtered or centrifuged and dried to obtain calcium carbonate powder ( Figure 5 ).
[0038] Table 1 SEM images of calcium carbonate powder obtained under various conditions
[0039] Figure 5
[0040] The particle morphology of the obtained calcium carbonate powder is as Figure 5 As shown, the calcite crystals of rh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com