Cation antibacterial peptides, their preparation method and application
An ion exchange chromatography, cyclic peptide technology, applied in the preparation method of peptides, antibacterial drugs, antifungal agents, etc., can solve the problems of high antibacterial activity and low hemolytic activity, and achieve the effect of significant bactericidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: Preparation and purification of AMP-1
[0046] Sequence: Phe-Leu-Phe-Leu-Phe-Leu-Lys-Lys-Arg-Lys-Lys-Arg-Lys-His-NH 2 (SEQ ID NO: 1)
[0047] (1) Materials and reagents
[0048] Rink Amide MBHA resin, substitution value 0.41mmol / g.
[0049] The amino acids to be protected are Fmoc-L-Arg(Pbf)-OH, Fmoc-L-Leu-OH, Fmoc-L-Lys(Boc)-OH, Fmoc-L-Phe-OH and Fmoc-L-His (Trt)-OH.
[0050] Reagents: HOBt, DIC, DMF, piperidine.
[0051] (2) Instrument
[0052] PSI300 peptide synthesizer, Waters600 semi-preparative high performance liquid chromatography, magnetic stirrer.
[0053] (3) Operation steps (take 0.25mmol as an example)
[0054] a. Solid-phase chemical synthesis of peptides
[0055] Weigh 0.61g of Rink Amide MBHA resin, put it in the reactor of the peptide synthesizer, add 10mL of DMF, soak for 2h, then add 15mL of 20% PIP / DMF solution, mix for 30min to remove the amino protecting agent, and wash the resin 7 times with DMF , then add 619.8mg Fmoc-L-His...
Embodiment 2
[0070] Embodiment 2: Preparation and purification of AMP-2 and AMP-3
[0071] Sequence: Phe-Leu-Leu-Phe-Leu-Leu-Lys-Lys-Arg-Lys-Lys-Arg-Lys-His-NH 2 (SEQ ID NO: 2)
[0072] Trp-Trp-Trp-Phe-Trp-Trp-Lys-Lys-Arg-Lys-Lys-Arg-Lys-His-NH 2 (SEQ ID NO: 3)
[0073] (1) Materials and reagents
[0074] Rink Amide MBHA resin, substitution value 0.41mmol / g.
[0075] Required protected amino acids Fmoc-L-Arg(Pbf)-OH, Fmoc-L-Leu-OH, Fmoc-L-Lys(Boc)-OH, Fmoc-L-Phe-OH, Fmoc-L-Trp( Boc)-OH and Fmoc-L-His(Trt)-OH.
[0076] Reagents: HOBt, DIC, DMF, piperidine.
[0077] (2) Instrument
[0078] PSI300 peptide synthesizer, Waters600 semi-preparative high performance liquid chromatography, magnetic stirrer.
[0079] (3) Operation steps (take 0.25mmol as an example)
[0080] AMP-2 and AMP-3 were prepared and purified in a manner similar to the steps a-c in Example 1, and the fractions with a purity greater than 99% were collected and then concentrated to dryness at 50°C under reduced pressu...
Embodiment 3
[0082] Embodiment 3: Preparation and purification of cationic antimicrobial peptides in table 2
[0083] The cationic antimicrobial peptides in Table 4 were prepared and purified in a similar manner to Example 1, but the cationic antimicrobial peptides involved in the present invention are not limited thereto.
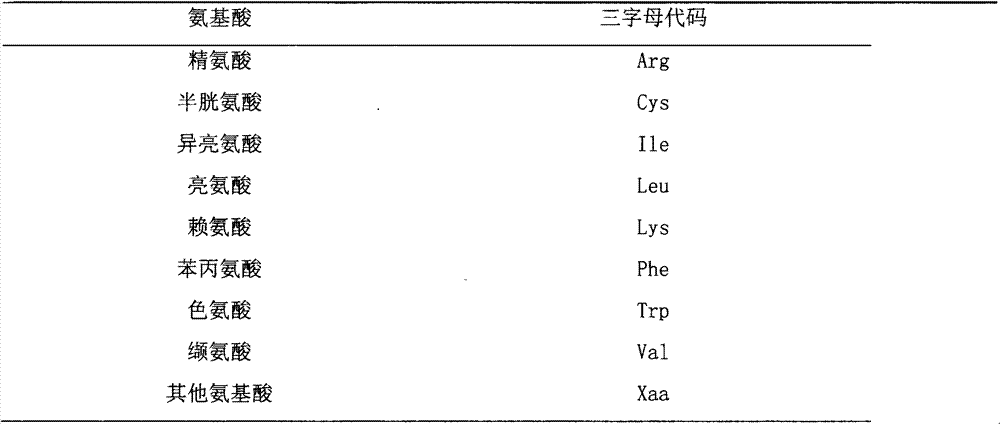
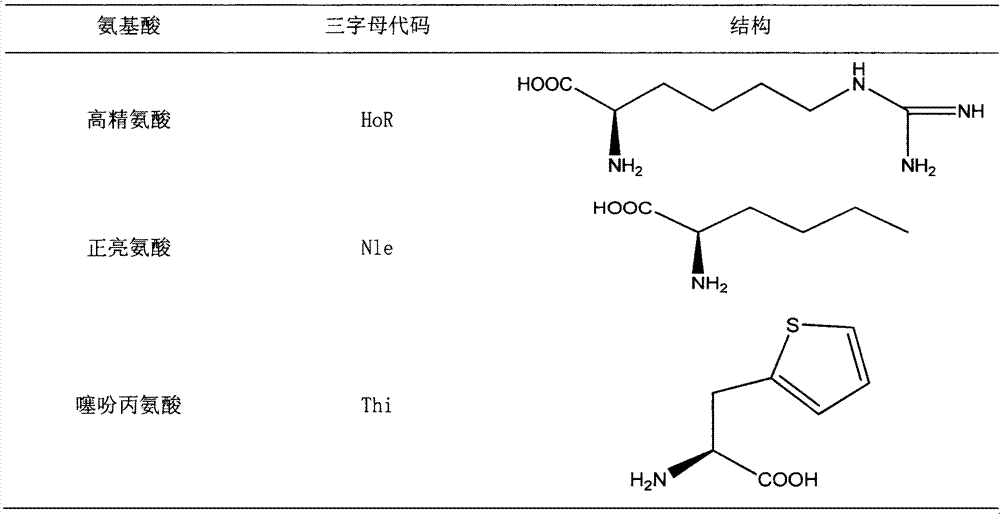
[0084] Table 4 Cationic Antimicrobial Peptides
[0085]
[0086]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com