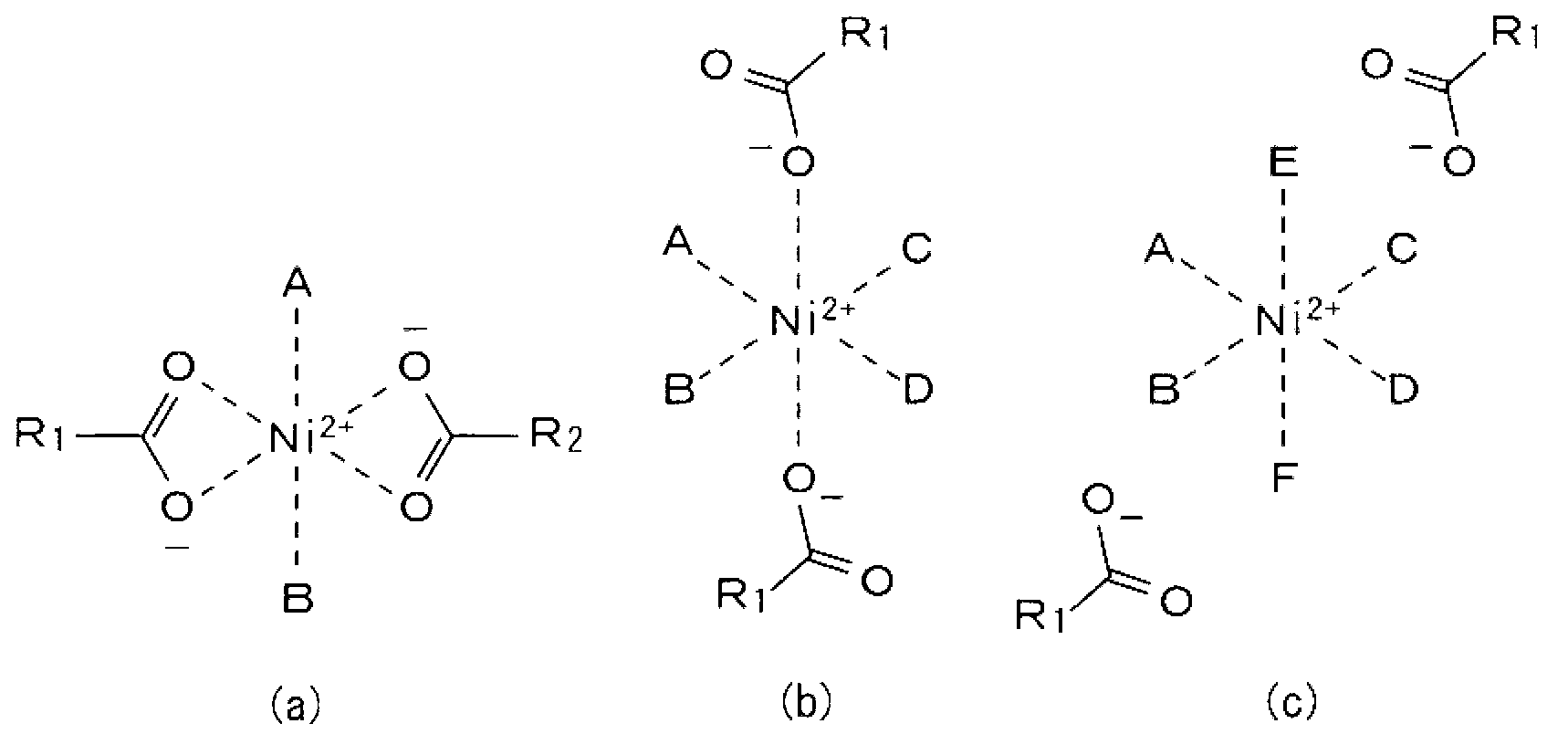
Nickel-cobalt nanoparticle and manufacturing method therefor
A manufacturing method, cobalt nanotechnology, applied in the direction of structural parts, transportation and packaging, coating, etc., can solve unclear and difficult problems, and achieve the effect of reasonable cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] 275 mmol of oleylamine was added to 12.5 mmol of cobalt formate dihydrate and 12.5 mmol of nickel acetate tetrahydrate, and heated at 120° C. for 20 minutes under a nitrogen flow to obtain a complex reaction liquid. Next, the complex reaction solution was heated to 225° C. with microwaves, and the temperature was maintained for 30 minutes to obtain a nanoparticle slurry. The nanoparticle slurry was separated by standing, and the supernatant was removed, followed by washing with hexane three times. After that, it was dried with a vacuum dryer maintained at 60° C. for 6 hours to obtain nanoparticles.
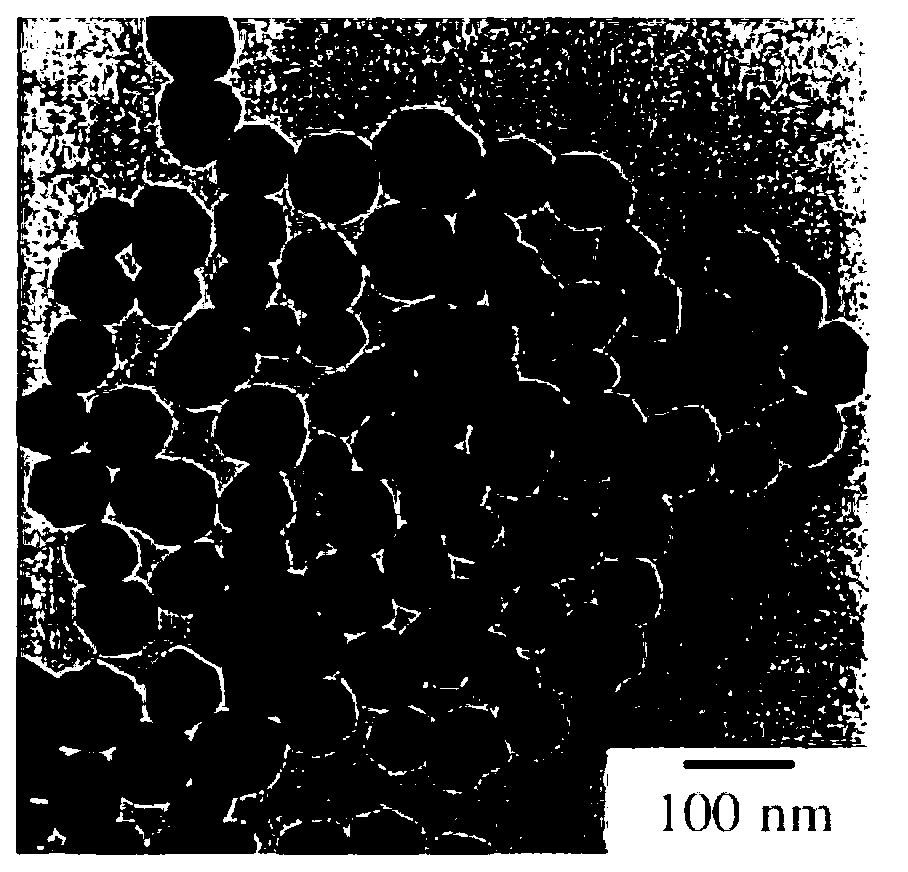
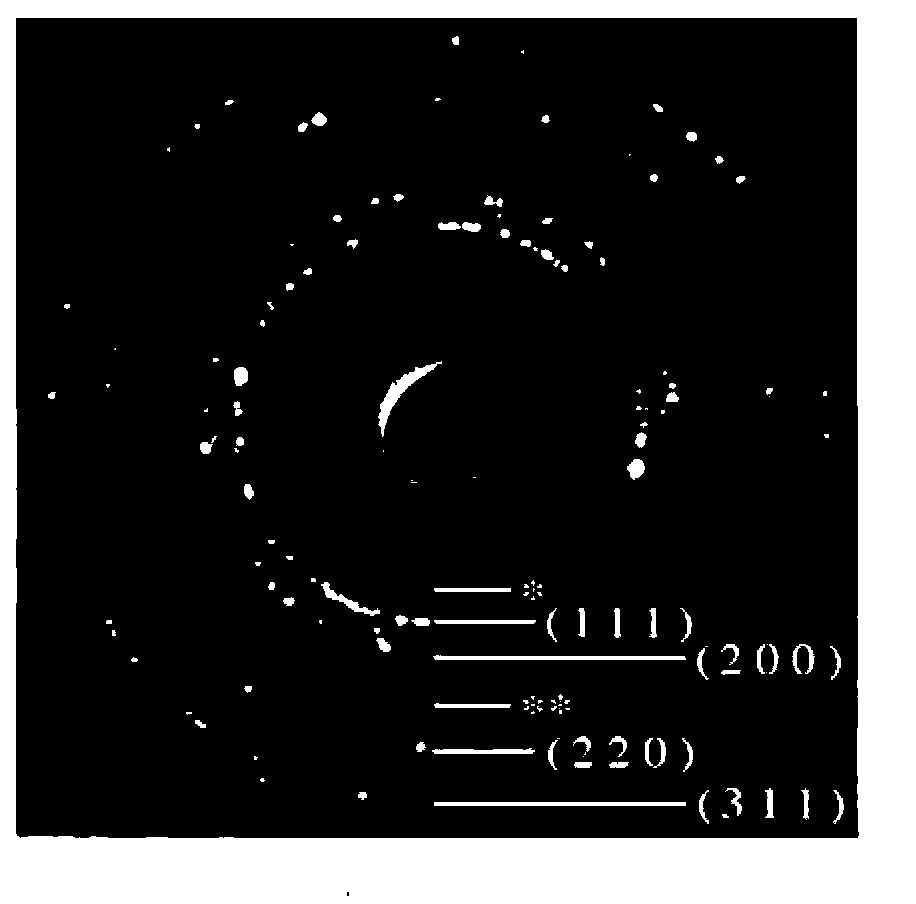
[0098] A TEM (Transmission Electron Microscope, transmission electron microscope) photograph of the obtained nanoparticles is shown in Figure 2A , the ED (Electron diffraction: Electron diffraction) pattern is shown in Figure 2B . Spherical uniform particles with an average particle diameter of 82 nm were formed. In addition, from the ED pattern, it can be seen that t...
Embodiment 2~7、 comparative example 1~8
[0101] In Examples 2-7 and Comparative Examples 1-8, except changing the kind of nickel salt and cobalt salt and the kind of heating source and the reaction temperature (heating temperature) in the process of obtaining nanoparticle slurry, according to Example 1 to prepare nanoparticles. The results are shown in Table 1 together with Example 1. In addition, the molar ratio of oleylamine / (Ni salt+Co salt) was 10 in each of Examples and Comparative Examples. In addition, with respect to the heating method of the complexation reaction liquid, except Example 3 which used an oil bath, microwave heating was performed for all.
[0102] In addition, the results of the SQUID magnetic susceptibility measurement of the nickel-cobalt nanoparticles of Examples 1 and 2, the nickel particles of Comparative Example 8, and commercially available cobalt particles are shown in Figure 5 , the TEM photo of the nickel-cobalt nanoparticles obtained in Example 2 is shown in Figure 6 , the TEM ph...
Embodiment 8
[0107] In 12.5 mmol of cobalt formate dihydrate and 25 mmol of nickel particles with an average particle size of 100 nm and a Cv value of 0.14 (without using cobalt salts, the complexation reaction was obtained from nickel acetate tetrahydrate according to the first example above, which was heated to form The prepared particles; Comparative Example 8) were added with 125 mmol of oleylamine, and heated at 120° C. for 20 minutes under a nitrogen stream to obtain a complex reaction solution. Next, the complex reaction solution was heated to 225° C. with microwaves, and the temperature was maintained for 30 minutes to obtain a nickel-cobalt nanoparticle slurry.
[0108] The above-mentioned nickel-cobalt nanoparticle slurry was separated by standing, and after removing the supernatant, it was washed three times with hexane. Thereafter, it was dried with a vacuum dryer maintained at 60° C. for 6 hours to obtain nickel-cobalt nanoparticles. The average particle diameter of the obtai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com