Macrocyclic Ghrelin Receptor Antagonists And Inverse Agonists And Methods Of Using The Same
A six-membered ring and five-membered ring technology, applied in the field of macrocyclic compounds, can solve the problems of poor selectivity and limited
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
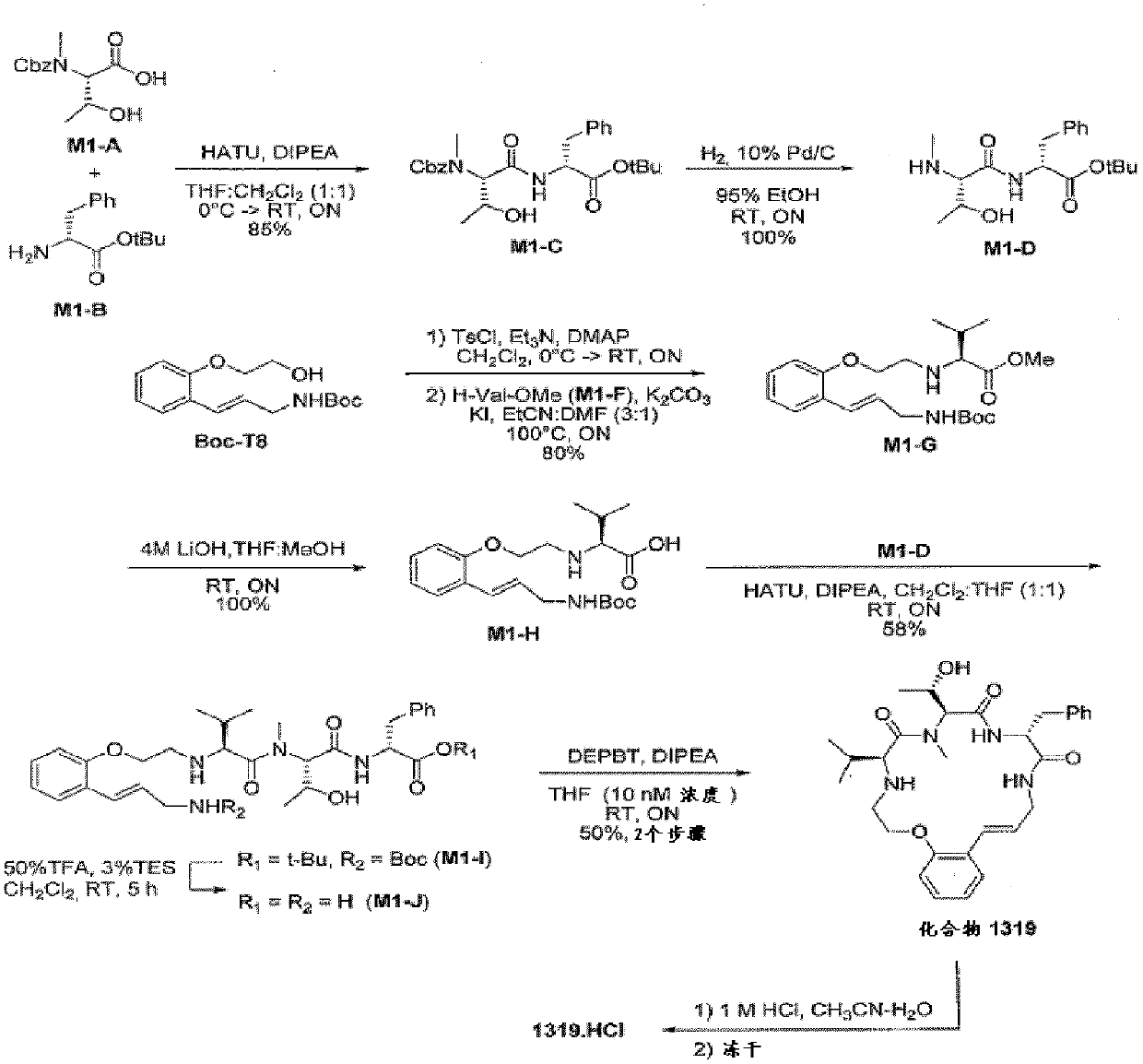
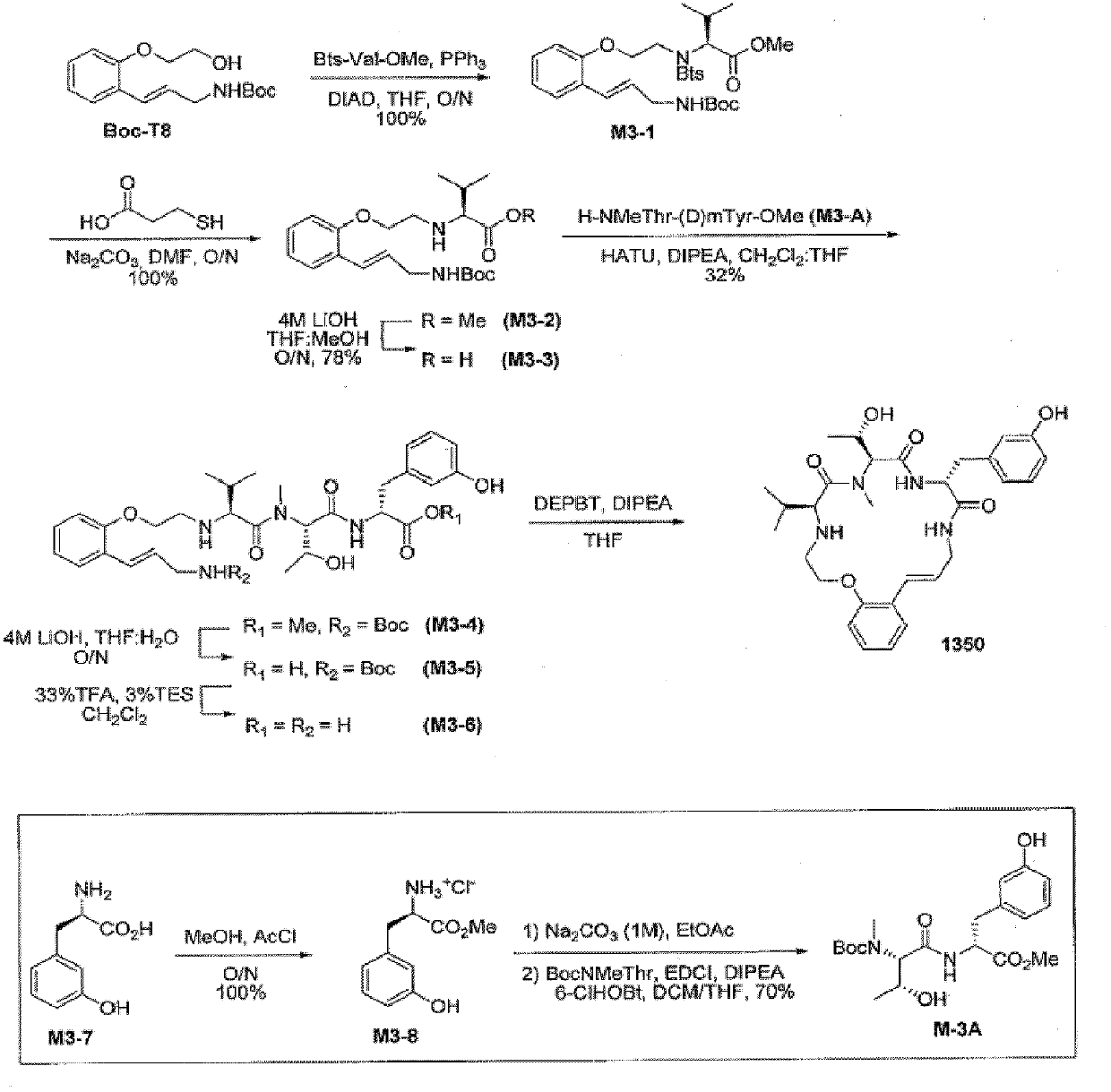
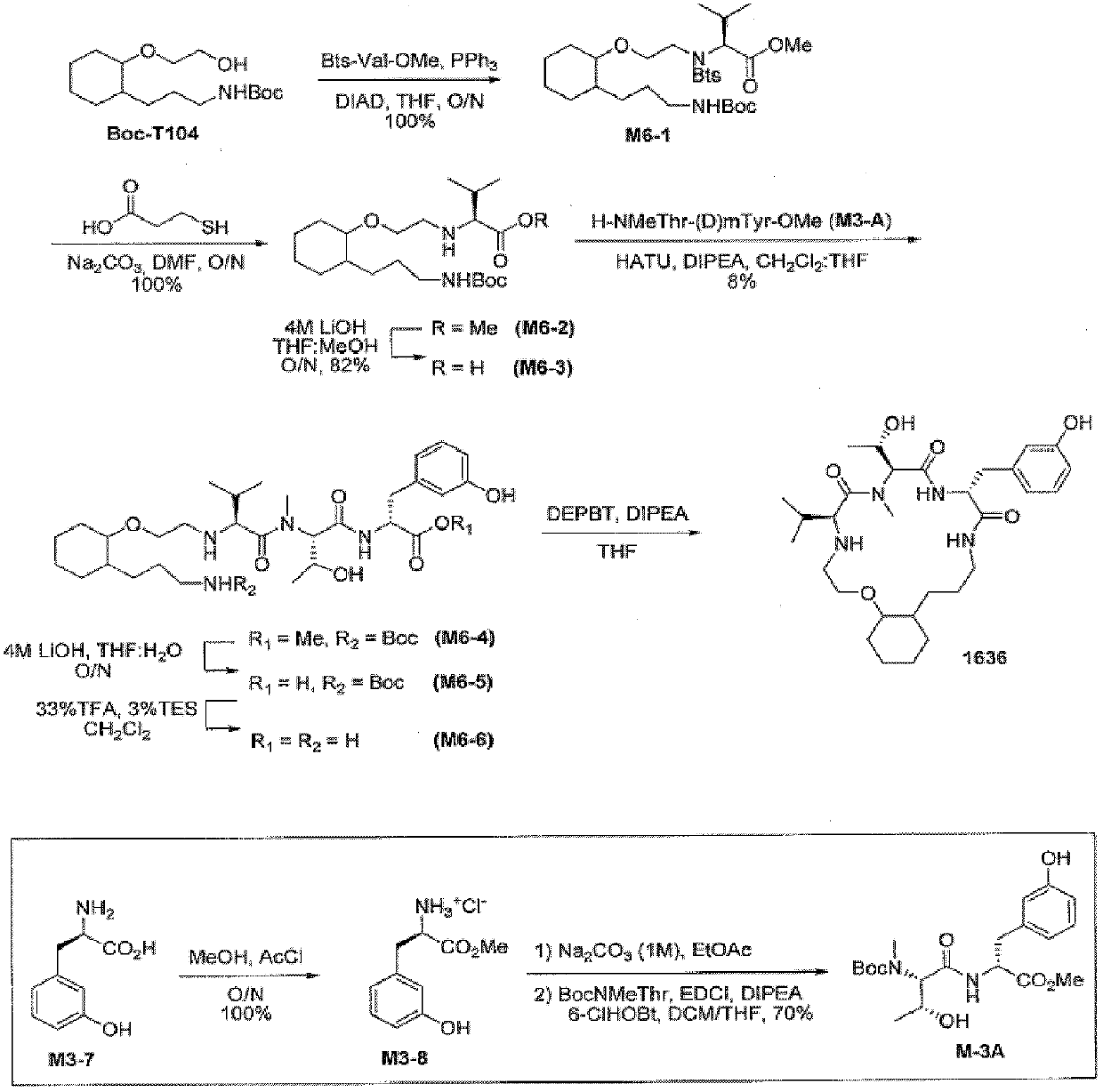
[0671] amino acid building block
Embodiment AA1
[0672] Example AA1. Standard Procedure for the Synthesis of H-(3Me)Cpg-OH
[0673]
[0674] Step AA1-1: Cyclopropanation. To a solution of 3-methyl-3-buten-1-ol (AA1-A, 3.52 mL, 34.8 mmol, 1.0 equiv) in DCM (350 mL) was carefully added neat diethyl at -20 °C under argon atmosphere Zinc (17.9 mL, 174 mmol, 5.0 equiv) and diiodomethane (28.1 mL, 348 mmol, 10.0 equiv) were added and the temperature was rapidly raised to 0 °C. (Note: Temperature control is very important. Diiodomethane (melting point: 5-8°C) and diethylzinc (melting point: -28°C) can freeze suddenly and stop stirring, and there is a risk of explosion when melting). The reaction was allowed to warm slowly to room temperature and stirred overnight. Add saturated NH to the mixture 4 Cl (aq), and with Et 2 The aqueous phase was extracted with O (3x). with saturated NaHCO 3 The combined organic phases were washed with aqueous solution (2×), brine (1×), washed over MgSO 4 Dry, filter, and then concentrate the ...
Embodiment AA2
[0681] Example AA2: Standard procedure for the synthesis of H-trans-(3H,4Me)Cpg-OH
[0682]
[0683] Step AA2-1: Cyclopropanation. To a solution of (Z)-pent-3-en-1-ol (AA2-A, 3.34 g, 38.9 mmol, 1.0 equiv) in DCM (390 mL) was carefully added neat diethylzinc (20.0 mL, 194 mmol, 5.0 equiv) and diiodomethane (31.4 mL, 398 mmol, 10.0 equiv), and the temperature was rapidly raised to 0°C. (Note: Temperature control is very important. Diiodomethane (melting point: 5-8°C) and diethylzinc (melting point: -28°C) can freeze suddenly and stop stirring, and there is a risk of explosion when melting). The reaction was allowed to warm slowly to room temperature and stirred overnight. Add saturated NH 4 Cl(aq) with Et 2 The aqueous phase was extracted with O (3x). with saturated NaHCO 3 The combined organic phases were washed with aqueous solution (2×), brine (1×), washed over MgSO 4 Dried, filtered, and concentrated by rotary evaporator at low temperature and low pressure (due to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com