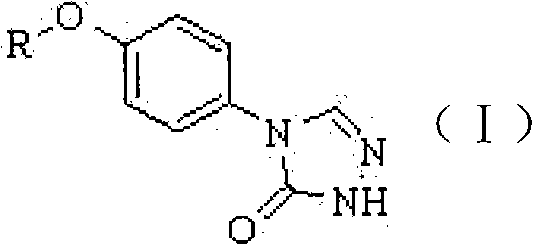
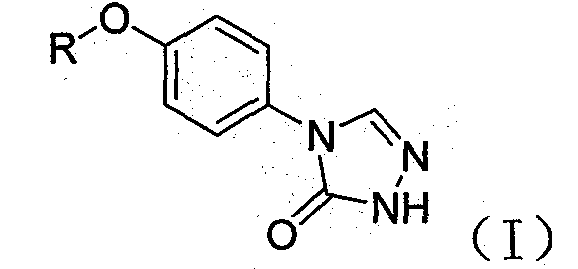
A class of compounds and pharmaceutically acceptable salts thereof as antiepileptic drugs
A technology of antiepileptic drugs and compounds, applied in the field of preparation of antiepileptic drugs, capable of solving problems such as side effects and discomfort
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
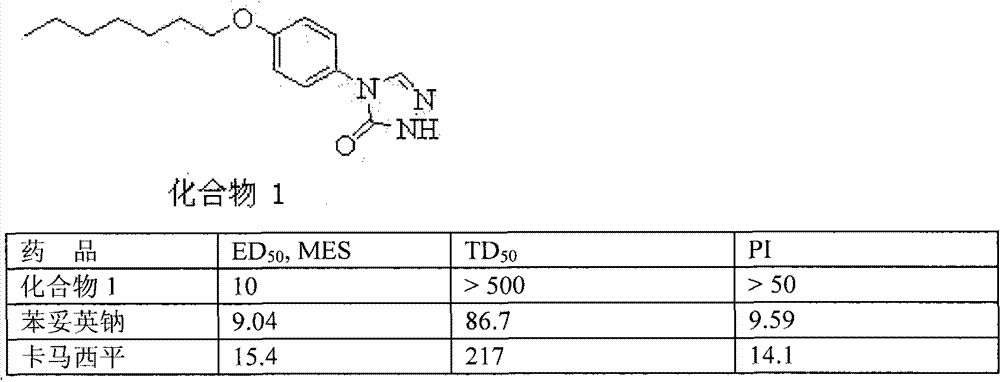
[0013] Example A: Anticonvulsant Pharmacological Experiment and Neurotoxicity Experiment
[0014] All compounds provided herein were screened for their ability to prevent and treat electrically induced epilepsy in epilepsy. Maximal electroshock MES test, used to show the efficacy of antiepileptic drugs against generalized seizures. The compounds of the invention can be used to inhibit or prevent convulsions by oral (P.O) administration to mice.
[0015] A rotarod ataxia test was also performed on mice to evaluate the neurotoxicity of each of the claimed agents, measuring TD 50 And the protection index PI. Using mice, the biological activity and neurotoxicity of the test compounds in the maximum electroshock (MES) test seizure threshold test were tested. See Krall, R.J.; Penry, J.K.; White, B.G.; Kupferberg, H.J.; Swinyard, E.A. Epilepsia. 1978, 19, 409.
[0016] Table 1. Results of anticonvulsant pharmacological experiments and neurotoxicity experiments (mg / kg, p.o)
[001...
Embodiment B
[0018] Embodiment B: pharmaceutical composition
[0019] Formulation for 1000 tablets each containing 100 mg of active ingredient:
[0020] 4-(4-heptyloxy)phenyl-2H-1,2,4-triazol-3(4H)one-------------100g
[0021] Hydroxypropyl cellulose --------------------------------------- 2g
[0022] Wheat starch ------------------------------------------ 10g
[0023] Lactose ----------------------------------------- 100g
[0024] Magnesium stearate ------------------------------------------3g
[0025] Talc -------------------------------------------- 3g
[0026] The dosage used should be adapted to the nature and severity of the disease, the route of administration and the age and weight of the patient. The daily dose varies between 0.01mg-1g, and can be administered once or in fractions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com