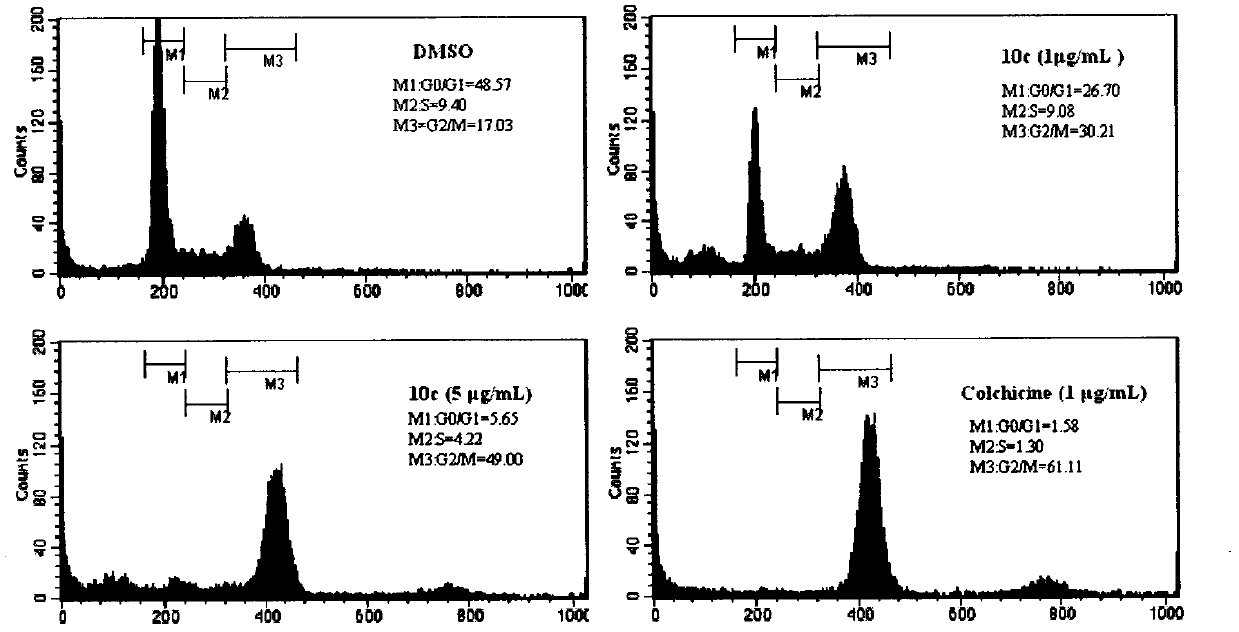
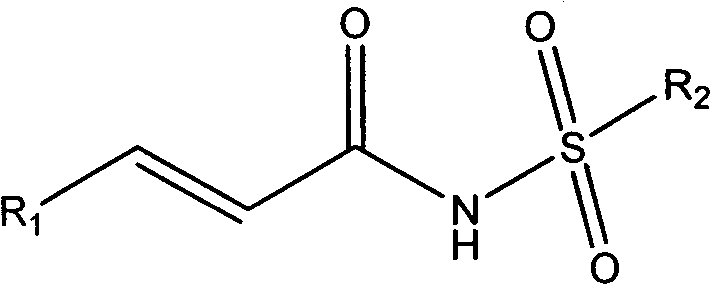
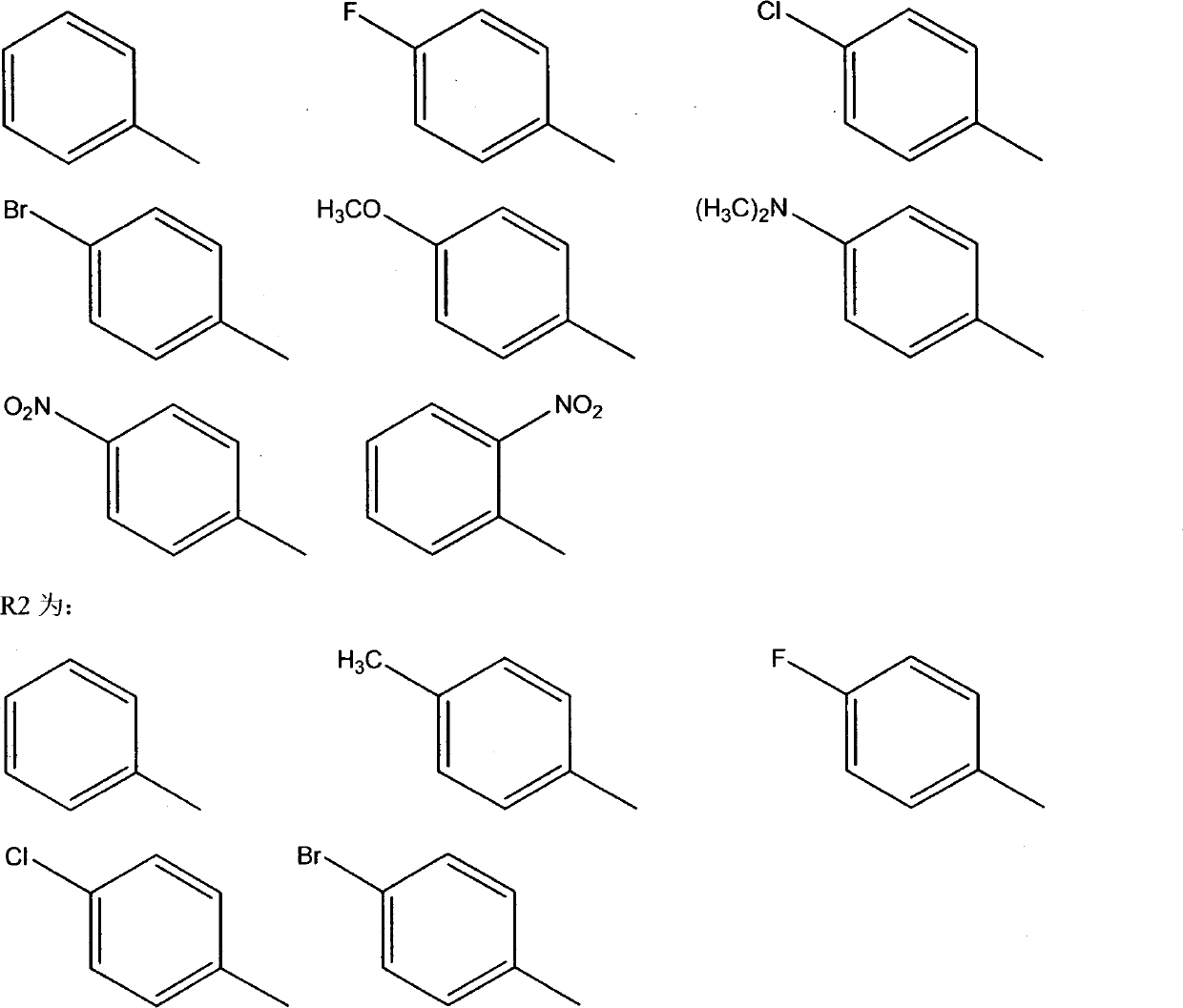
Cinnamoyl sulfonamide compound preparation and applications of cinnamoyl sulfonamide compounds in anti-tumor treatment drugs
A technology of cinnamoyl sulfonamides and compounds, which is applied in the preparation of cinnamoyl sulfonamide compounds and their application in anticancer drugs, and can solve the problems of sulfonamide biological activity, low cytotoxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: Preparation of N-(p-fluorobenzenesulfonyl)cinnamide (compound 9c)
[0016]
[0017] In a 100ml one-necked flask, dissolve benzaldehyde (10mmol) and malonic acid (12.1mmol) in 30ml of pyridine respectively, stir magnetically and heat to 80-90°C, add piperidine (1.21mmol) dropwise and continue heating and stirring for 24h (TLC detects the degree of progress of the reaction). After the reaction was completed, the solvent was dried with a vacuum pump, and the residual solid was washed with 10% hydrochloric acid. After fully mashing, filter with suction and wash with a large amount of water, and finally wash with n-hexane for 3 times, and dry to obtain the product cinnamic acid. The product was purified, after drying, weighed 2g and added it to another 100ml single-necked flask, added an equimolar amount of p-fluorobenzenesulfonamide, and added 2.2 times the molar amount of EDCI and DMAP, and used 20ml CH 2 Cl 2 Dissolve, and react with magnetic stirring at ...
Embodiment 2
[0018] Example 2: Preparation of N-(p-bromobenzenesulfonyl)cinnamide (compound 9e)
[0019]
[0020] The preparation method is the same as in Example 1. Replace p-fluorobenzenesulfonamide in Example 1 with p-bromobenzenesulfonamide. The target compound was obtained as colorless crystals. Yield 90%; m.p.166-167℃. 1 H NMR (300MHz, CDCl 3 ): δ6.39 (d, J=15.57Hz, 1H), 7.35-7.42 (m, 3H), 7.44-7.50 (m, 2H, ArH), 7.67-7.72 (dd, J 1 = 3.3Hz,J 2 =12.06Hz, 3H, ArH), 8.00 (d, J=8.79Hz, 2H, ArH), 8.49 (s, 1H, NH).ESI-MS: 365.97 (C 15 h 13 BrNO 3 S, [M+H] + ).Anal.Calcd for C 15 h 12 BrNO 3 S: C, 49.19; H, 3.30; N, 3.82%. Found: C, 49.35; H, 3.46; N, 3.92%.
Embodiment 3
[0021] Example 3: Preparation of (E)-3-(4-fluorophenyl)-N-(benzenesulfonyl)acrylamide (compound 10a)
[0022]
[0023] The preparation method is the same as in Example 1. Replace the benzaldehyde in the example one with p-fluorobenzaldehyde, and replace the p-fluorobenzenesulfonamide in the example one with benzenesulfonamide. The target compound was obtained as pale yellow crystals. Yield 82%; m.p.165-166℃. 1 H NMR (300MHz, CDCl 3 ): δ6.34 (d, J=15.54Hz, 1H), 7.04-7.10 (m, 2H, ArH), 7.45-7.50 (m, 2H), 7.54-7.57 (m, 2H, ArH), 7.59-7.68 (m, 2H, ArH), 8.10-8.17 (m, 3H).ESI-MS: 306.05 (C 15 h 13 FNO 3 S, [M+H] + ).Anal.Calcd for C 15 h 12 FNO 3 S: C, 59.01; H, 3.96; N, 4.59%. Found: C, 59.23; H, 4.23; N, 4.75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com