Prochiral allyl amine compound and synthetic method thereof
A technology of allyl amine and synthesis method, which is applied in the field of synthesis of prochiral allyl amine compounds, can solve the problems of low yield, poor reaction selectivity, difficult procurement, etc., and achieve target yield and purity improvement, The effect of economical and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
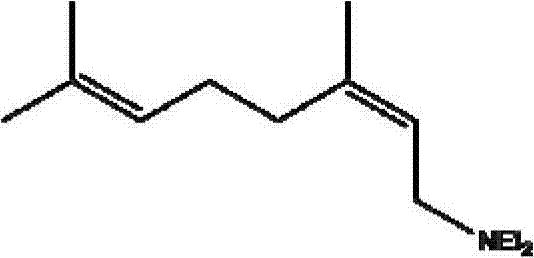
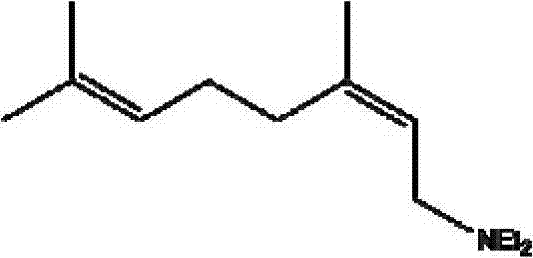
[0022] Nerylamine, its structural formula is:
[0023]
[0024] The synthetic method of neroliamine comprises the steps:
[0025] In a dry 1-liter high-pressure reaction device, under the protection of nitrogen, add 340g of isoprene, 80g of diethylamine, 100ml of toluene, after stirring evenly, add 26ml of 2.5M n-hexane mixed solution of butyllithium, seal and Pressurize the kettle, control the reaction temperature at 70°C and the reaction pressure within the range of 80psi, react for 12 hours, after the reaction is completed, cool to room temperature, release the pressure and transfer the materials in the reactor to ice water, and separate the two phases at rest , the lower aqueous phase was extracted with methyl tert-butyl ether and incorporated into the organic phase, washed with saturated brine, dried over anhydrous sodium sulfate, and rectified. The obtained content is 99.0% of neroliamine, and the molar yield is 82%.
Embodiment 2
[0027] Nerylamine, its structural formula is:
[0028]
[0029] In a dry 1-liter high-pressure reaction device, under the protection of nitrogen, add 340g of isoprene, 80g of diethylamine, and 50ml of n-hexane. After stirring evenly, put 0.6g of pre-cut metal lithium particles into the kettle under stirring. Seal the device and pressurize the autoclave, control the reaction temperature within the range of 120°C and reaction pressure 140psi, and react for 24 hours. phase, the lower aqueous phase was extracted with ether and incorporated into the organic phase, washed with saturated brine, dried over anhydrous sodium sulfate, and rectified to obtain 176 g of nerylamine with a content of 99.5% and a molar yield of 90%.
Embodiment 3
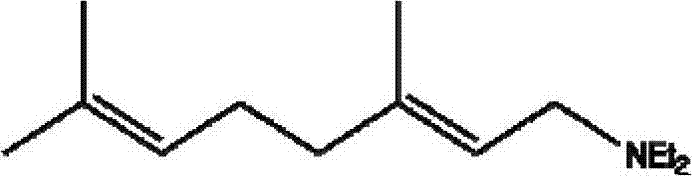
[0031] Hazy bovine diamine, its structural formula is:
[0032]
[0033] In a dry 1-liter high-pressure reaction device, under the protection of nitrogen, add 136g of myrcene, 100g of diethylamine, and 200ml of cyclohexane. After stirring evenly, add 67ml of 1.5M tetrahydrofuran solution of butyllithium, and control the reaction temperature at Within the range of 65°C and reaction pressure 70psi, react for 4 hours, cool the reaction solution to room temperature, put it into ice water, and separate the two phases at rest. The lower aqueous phase is extracted with dichloromethane and incorporated into the organic phase, washed with saturated saline, Dried over sodium sulfate and rectified to obtain 97g of 99.3% oxadiamine, 25g of 96% oxadiamine, and 40g of 89% oxadiamine with a molar yield of 80%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap