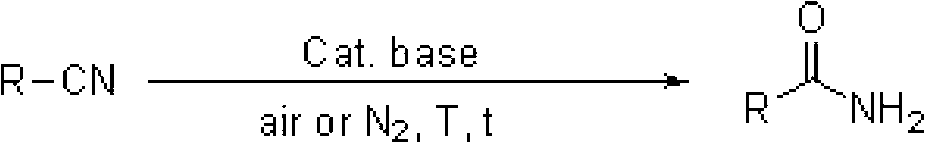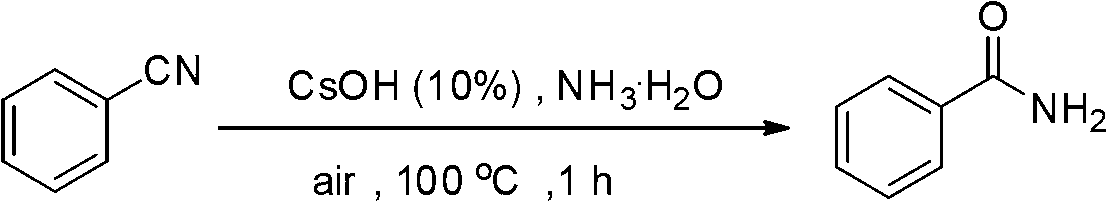Preparation method of amide from nitrile
An amide and catalyst catalyzing nitrile technology, which is applied in the formation/introduction of amide groups, the preparation of carboxylic acid amides, chemical instruments and methods, etc., can solve the problems of high toxicity, poor catalyst effect, and large amount of catalyst.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Preparation of benzamide from benzonitrile
[0018]
[0019] Add CsOH·H to the reaction tube sequentially 2 O (0.0336g, 10mol%) and PhCN (2mmol), and ammonia water (1.0mL) was added as a solvent, and the reaction tube was sealed and heated to 100°C for 1h. The reaction conversion rate was over 99% as measured by GC-MS, and the product was separated and purified by column chromatography with a separation yield of 81%. 1 H NMR (500MHz, d 6 -DMSO): δ7.98(b,1H),7.89-7.86(m,2H),7.54-7.51(m,1H),7.47-7.44(m,2H),7.36(b,1H). 13 C NMR (125.4MHz, d 6 -DMSO): δ167.9, 134.2, 131.2, 128.2, 127.4. MS (EI): m / z (%) 224 (6), 223 (32), 222 (14), 132 (2), 131 (8), 130(4),118(2),106(23),105(100),104(5),103(9),91(5),79(9),78(4),77(11), 65(3).
Embodiment 2
[0021] Preparation of p-toluamide from p-methylbenzonitrile
[0022]
[0023] Add CsOH·H to the reaction tube sequentially 2 O (0.0336g, 10mol%) and p-tolunitrile (2mmol), and ammonia water (1.0mL) was added as a solvent, and the reaction tube was sealed and heated to 100°C for 6h. The reaction conversion rate was over 99% as measured by GC-MS, and the product was separated and purified by column chromatography with a separation yield of 80%. 1 H NMR (500MHz, CDCl 3 ):δ7.71(d,J=8.0Hz,2H),7.25(d,J=8.0Hz,2H),6.01(b,2H),2.40(s,3H). 13 C NMR (125.4MHz, CDCl 3 ):δ169.4,142.5,130.6,129.3,127.4,21.5.MS(EI):m / z(%)136(5),135(60),120(8),119(100),117(3), 92(5),91(74),90(8),89(10),65(33),64(4),63(13),51(9),50(6),44(12), 41(5), 40(9), 39(20), 38(4).
Embodiment 3
[0025] Preparation of m-methylbenzamide from m-methylbenzonitrile
[0026]
[0027] Add CsOH·H to the reaction tube sequentially 2 O (0.0336g, 10mol%) and m-tolunitrile (2mmol), and ammonia water (1.0mL) was added as a solvent, and the reaction tube was sealed and heated to 100°C for 6h. The reaction conversion rate was over 99% as measured by GC-MS, and the product was separated and purified by column chromatography with a separation yield of 68%. 1 H NMR (500MHz, d 6 -DMSO): δ7.91(b,1H),7.71(s,1H),7.68-7.66(m,1H),7.33(d,J=5.0Hz,2H),7.30(b,1H),2.35( s,3H). 13 C NMR (125.4MHz, d 6 -DMSO): δ168.0, 137.4, 134.3, 131.7, 128.0, 124.5, 20.9. MS (EI): m / z (%) 136 (6), 135 (63), 120 (9), 119 (100), 117 (4),116(2),92(6),91(77),90(5),89(7),65(18),63(6),62(2),51(4),44 (3), 39(6).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com