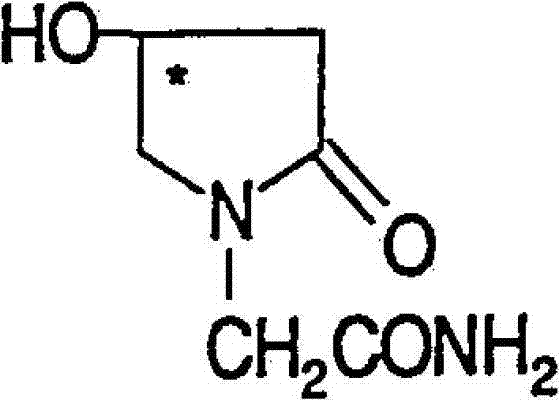
Pharmaceutical composition comprising (S)-4-hydroxy-2-oxo-1-pyrrolidine acetamide
A composition and drug technology, applied in the field of medicine, can solve the problems such as the inability of levoxiracetam to be applied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Embodiment 1: Preparation of S-oxiracetam freeze-dried powder injection
[0078] prescription:
[0079] components
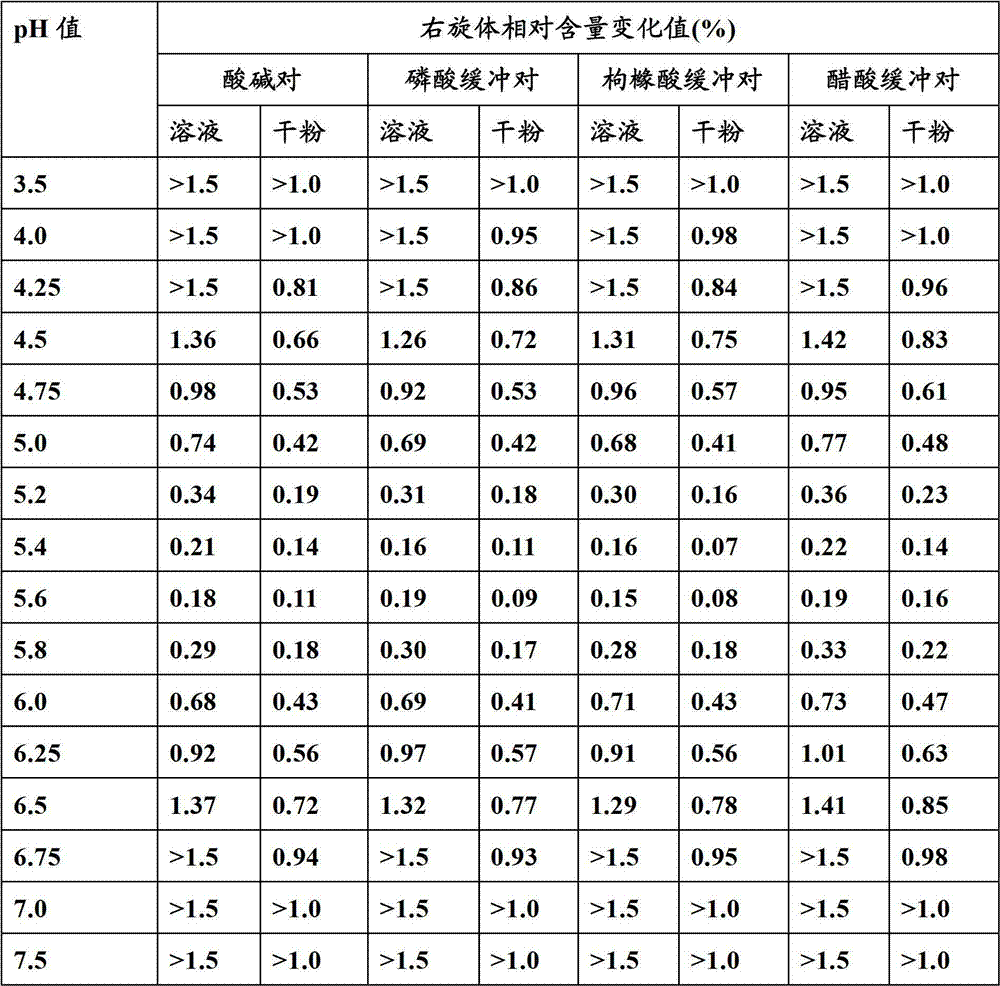
[0080]Preparation method: Take 80% of the prescribed amount of water for injection, add the active ingredient of the prescribed amount and other auxiliary materials and stir to dissolve, adjust the pH value to a suitable value with 1M hydrochloric acid solution and / or 1M sodium hydroxide solution (make it dilute with water for injection) When the active ingredient concentration is 1g / 20ml, the pH value is 5.5), add 0.1% activated carbon for injection according to the prepared amount, heat the liquid medicine to about 60°C, stir for 20min, filter and decarbonize, add water for injection to the total amount, Intermediate detection. After passing the test of the intermediate, it is fine-filtered with a 0.22 μm microporous membrane, filled into a vial, placed in a freeze dryer, and freeze-dried to obtain the product.
Embodiment 2
[0081] Example 2: Preparation of S-oxiracetam freeze-dried powder injection
[0082] prescription:
[0083] components
Dosage
S-oxiracetam
1000g
2g
Water for Injection
Add to 2000ml
Co-made
1000 bottles
[0084] Wherein the relative content of the dextrorotatory form in the raw material S-oxiracetam is 0.36%.
[0085] Preparation method: Take 80% of the prescribed amount of water for injection, add the active ingredient of the prescribed amount and other auxiliary materials and stir to dissolve, adjust the pH value to a suitable value with 1M hydrochloric acid solution and / or 1M sodium hydroxide solution (make it dilute with water for injection) When the concentration of the active ingredient is 1g / 20ml, the pH value is 5.2), add 0.1% activated carbon for injection according to the prepared amount, heat the liquid medicine to about 60°C, stir for 20min, filter and decarbonize, add water...
Embodiment 3
[0086] Example 3: Preparation of S-oxiracetam freeze-dried powder injection
[0087] prescription:
[0088] components
Dosage
S-oxiracetam
1000g
100g
Water for Injection
Add to 10000ml
Co-made
1000 bottles
[0089] Wherein the relative content of the dextrorotatory form in the raw material S-oxiracetam is 0.36%.
[0090] Preparation method: Take 80% of the prescribed amount of water for injection, add the active ingredient of the prescribed amount and other auxiliary materials and stir to dissolve, adjust the pH value to a suitable value with 1M hydrochloric acid solution and / or 1M sodium hydroxide solution (make it dilute with water for injection) When the active ingredient concentration is 1g / 20ml, the pH value is 5.8), add 0.1% activated carbon for injection according to the prepared amount, heat the liquid medicine to about 60°C, stir for 20min, filter and decarbonize, add water for injection to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com