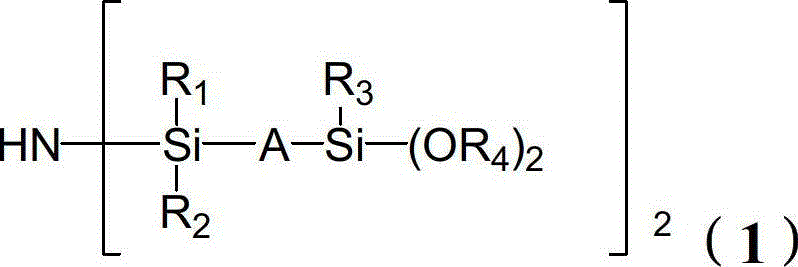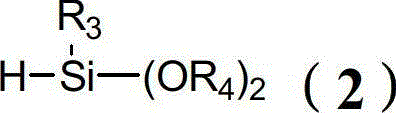Hydroxyl scavenging compound and preparation method and purpose thereof
A compound and alkyl technology, which is applied in the field of hydroxyl scavenger, can solve the problems of reducing the chemical bond between the filler surface and the polymer, and the deterioration of the mechanical properties of silicone rubber, so as to improve the tear resistance, mechanical properties, and storage stability. Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] The preparation of formula (1-1) compound (wherein R 1 = R 2 = R 3 = n-pentadecyl, R 4 = phenyl-substituted n-tetradecyl, A = -CH 2 CH 2 OCH 2 CH 2 -)
[0083]
[0084] Add 105.7g and 163.6g of
[0085]
[0086] Gently shake the flask to allow the reactants to mix evenly, slowly heat the mixture to 50°C, then drop 4g of chloroplatinic acid-isopropanol solution (the concentration of platinum in this chloroplatinic acid-isopropanol solution is 1000 μg / g ; Based on the total mass of reactants, the concentration of platinum is about 15 μg / g). The reaction was exothermic violently, and when the exotherm ceased (about 15 min), the reaction was terminated. Afterwards, use a vacuum pump to directly evacuate the obtained mixture to a vacuum degree of less than -0.098MPa, and remove the low boilers. The yield of the resulting product is 85%, and the characterization data are as follows:
[0087] 1 H-NMR (CDCl 3 , δppm): 0.96 (18H, t), 1.33 (12H, m), 1.29 (212H,...
Embodiment 2
[0089]The preparation of formula (1-2) compound (wherein R 1 =R 2 = Cyclohexyl substituted with ethoxy, R 3 =R 4 =n-butyl substituted cyclooctyl, A=-(CH 3 )CH-CH 2 -)
[0090]
[0091] Add 110.5g and 185.3g of
[0092]
[0093] Gently shake the flask to allow the reactants to mix evenly, slowly heat the mixture to 70°C, then drop 8g of chloroplatinic acid-isopropanol solution (the concentration of platinum in this chloroplatinic acid-isopropanol solution is 1000 μg / g ; Based on the total mass of the reactants, the concentration of platinum is about 27 μg / g), the reaction is violently exothermic, and when the exothermic stops (about 15min), the reaction ends. Afterwards, use a vacuum pump to directly evacuate the obtained mixture to a vacuum degree of less than -0.098MPa, and remove the low boilers. The yield of the resulting product is 80%, and the characterization data are as follows:
[0094] 1 H-NMR (CDCl 3 , δppm): 0.96(18H, t), 1.33(12H, m), 1.29(12H, m), ...
Embodiment 3
[0096] The preparation of formula (1-3) compound (wherein R 1 =R 2 = naphthyl, R 3 = n-nonyl, R 4 = n-octyl substituted by phenyl, A=-(CH 2 ) 2 -CH(OCH 3 )CH(CH 3 )CH 2 -)
[0097]
[0098] Add 135.2g and 188.7g of
[0099]
[0100] Gently shake the flask to allow the reactants to mix evenly, slowly heat the mixture to 70°C, then drop 5g of chloroplatinic acid-isopropanol solution (the concentration of platinum in this chloroplatinic acid-isopropanol solution is 1000 μg / g ; Based on the total mass of the reactants, the concentration of platinum is about 15.4 μg / g), the reaction is violently exothermic, and when the exothermic stops (about 15min), the reaction ends. Afterwards, use a vacuum pump to directly evacuate the obtained mixture to a vacuum degree of less than -0.098MPa, and remove the low boilers. The yield of the resulting product is 80%, and the characterization data are as follows:
[0101] 1 H-NMR (CDCl 3 , δppm): 0.96 (6H, t), 1.33 (4H, m), 1.29...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com