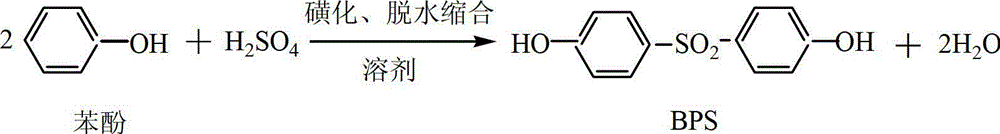
Method for preparing 4, 4'-dihydroxyl diphenyl sulfone
A technology of dihydroxydiphenyl sulfone and phenol, applied in the field of organic intermediate preparation, can solve the problems of difficult preparation of catalyst isophthalic acid, high price, etc., and achieves favorable dehydration reaction, high reaction temperature and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The material ratio is as follows:
[0028] The mass ratio of phenol to concentrated sulfuric acid is 1.93:1
[0029] The mass ratio of solvent to concentrated sulfuric acid is 2.07:1;
[0030] The mass ratio of catalyst 2,6-naphthalene disulfonic acid to concentrated sulfuric acid is 0.098:1
[0031] Add 106g of mesitylene and durene mixed solvent (the mass ratio of mesitylene to durene is 3:1) and 98.5g of phenol And catalyst 2,6-naphthalene disulfonic acid 5g. Stir the materials, and slowly add 51.1 g of concentrated sulfuric acid (concentration 98%) dropwise to the four-necked bottle, and the temperature reaches 110°C at the same time as the concentrated sulfuric acid is added dropwise in 2 hours. After the concentrated sulfuric acid was added dropwise, continue to heat up to the reflux temperature of 174° C., and maintain the reflux reaction temperature. The duration of this process is 8 hours. The reaction was considered complete after anhydrous evolution. Coo...
Embodiment 2
[0033] The material ratio is as follows:
[0034] The mass ratio of phenol to concentrated sulfuric acid is 1.89:1
[0035] The mass ratio of solvent to concentrated sulfuric acid is 1.85:1;
[0036] The mass ratio of catalyst 2,6-naphthalene disulfonic acid to concentrated sulfuric acid is 0.03:1
[0037] Add 94.5 g of mesitylene and durene mixed solvent (the mass ratio of mesitylene to durene is 4:1), phenol 96.6 g and 1.53 g of catalyst 2,6-naphthalene disulfonic acid. Stir the materials, and slowly add 51.1 g of concentrated sulfuric acid (concentration 98%) dropwise to the four-necked bottle, and it takes 2 hours for the concentrated sulfuric acid to be added dropwise. While adding concentrated sulfuric acid dropwise, start heating to 110°C, which also takes 2 hours. After the concentrated sulfuric acid was added dropwise, continue to heat up to the reflux temperature (172°C), and maintain the reflux reaction temperature. The duration of this process is 10h. The reac...
Embodiment 3
[0039] The material ratio is as follows:
[0040] The mass ratio of phenol to concentrated sulfuric acid is 2.03:1
[0041] The mass ratio of solvent to concentrated sulfuric acid is 2.48:1;
[0042] The mass ratio of catalyst 2,6-naphthalene disulfonic acid to concentrated sulfuric acid is 0.15:1
[0043] Add 126.7 g of mesitylene and durene mixed solvent (the mass ratio of mesitylene to durene is 2.8:1), phenol 103.7 g in a 500 mL four-neck flask equipped with reflux separator, thermometer and stirrer. g and 7.67 g of catalyst 2,6-naphthalene disulfonic acid. Stir the materials, and slowly add 51.1 g of concentrated sulfuric acid (concentration 98%) dropwise to the four-necked bottle, and it takes 2 hours for the concentrated sulfuric acid to be added dropwise. While adding concentrated sulfuric acid dropwise, start heating to 110°C, which also takes 2 hours. After the concentrated sulfuric acid was added dropwise, continue to heat up to the reflux temperature (175°C), a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com