Peptide complex, pharmaceutical composition thereof, and preparation and application thereof
A composition and drug technology, applied in the direction of drug combination, pharmaceutical formula, specific peptide, etc., can solve the problems of increasing the pain of patients and short half-life, and achieve the effect of prolonging the half-life and facilitating clinical promotion and application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Solid Phase Synthesis of Peptides
[0053] Using the solid-phase peptide synthesis method of the Fmoc strategy, the CS 336X instrument produced by CSBio Company was used to synthesize the peptide in the patent. The method of synthesis was carried out according to the manufacturer's instruction manual.
[0054] The prepared polypeptide was purified using HPLC C18 semi-preparative column, and the mobile phase was acetonitrile. The polypeptide freeze-dried powder is obtained by desalting and freeze-drying. The polypeptides included in this patent all contain disulfide bonds, and ammonium bicarbonate or other reducing agents are used to form disulfide bonds in the polypeptides.
Embodiment 2
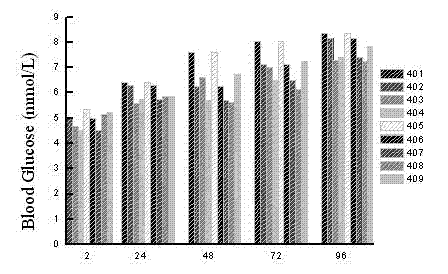
[0056] Hypoglycemic function related to GLP-1 analog (SEQ ID NO 4)
[0057] In this example, the polypeptides we use are as follows (401-427 corresponds to the sequence list 1-27):
[0058] SEQ ID NO 401: 7 HAEGT FTS C V SSYLE GQAAK EFIAW LVKGR G 37 GGGGG C GGG
[0059] SEQ ID NO 402: 7 HAEGT FTS C V SSYLE GQAAK EFIAW LVKGR G 37 GGGGG GGGGG C GGG
[0060] SEQ ID NO 403: 7 HAEGT FTS C V SSYLE GQAAK EFIAW LVKGR G 37 GGGGG GGGGG GGGGG C GGG
[0061] SEQ ID NO 404: 7 HAEGT FTS C V SSYLE GQAAK EFIAW LVKGR G 37 GGGGG C GGGGG G
[0062] SEQ ID NO 405: 7 HAEGT FTS C V SSYLE GQAAK EFIAW LVKGR G 37 GGGGG C GGGGG GGGG
[0063] SEQ ID NO 406: 7 HAEGT FTS C V SSYLE GQAAK EFIAW LVKGR G 37 GGGGG GGGGG C GGGGG G
[0064] SEQ ID NO 407: 7 HAEGT FTS C V SSYLE GQAAK EFIAW LVKGR G 37 GGGGG GGGGG C GGGGG GGGG
[0065] SEQ ID NO 408: 7 HAEGT FTS C V SSYLE GQAAK EFIAW LVKGR G 37 GGGGG GGGGG GGGGG C GGGGG G
[0066] SEQ ID NO 409: 7 HAEGT FTS C V SSYLE GQAAK ...
Embodiment 3
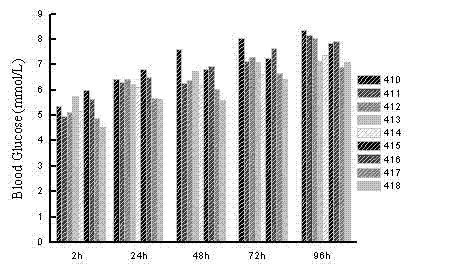
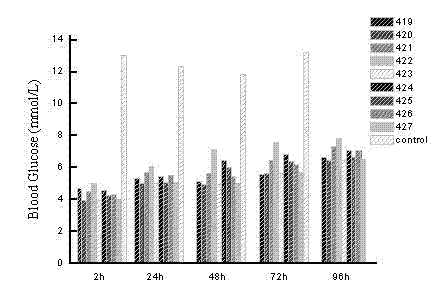
[0087] Hypoglycemic function associated with GLP-1 analog (SEQ ID NO 5). (Sequence Listing 28-55)
[0088] SEQ ID NO 501: 7 HAEGT FTSDV SSYLE GQAAK EFI C W LVKGR G 37 G C GGGGG GGGGG
[0089] SEQ ID NO 502: 7 HAEGT FTSDV SSYLE GQAAK EFI C W LVKGR G 37 G C GGGGG GGGGG GGGGG
[0090] SEQ ID NO 503: 7 HAEGT FTSDV SSYLE GQAAK EFI C W LVKGR G 37 G C GGGGG GGGGG GGGGG GGGGG GGGGG
[0091] SEQ ID NO 504: 7 HAEGT FTSDV SSYLE GQAAK EFI C W LVKGR G 37 GG C GGGGG GGGGG
[0092] SEQ ID NO 505: 7 HAEGT FTSDV SSYLE GQAAK EFI C W LVKGR G 37 GG C GGGGG GGGGG GGGGG
[0093] SEQ ID NO 506: 7 HAEGT FTSDV SSYLE GQAAK EFI C W LVKGR G 37 GG C GGGGG GGGGG GGGGG GGGGG
[0094] SEQ ID NO 507: 7 HAEGT FTSDV SSYLE GQAAK EFI C W LVKGR G 37 GGGGG C GGGGG GGGGG
[0095] SEQ ID NO 508: 7 HAEGT FTSDV SSYLE GQAAK EFI C W LVKGR G 37 GGGGG C GGGGG GGGGG GGGGG
[0096] SEQ ID NO 509: 7 HAEGT FTSDV SSYLE GQAAK EFI C W LVKGR G 37 GGGGG C GGGGG GGGGG GGGGG GGGGG
[0097]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com