Targeting gene transferring method of folic acid-functionalized PAMAM (polyamidoamine dendrimers) wrapped by gold nanoparticles
A technology for converting polyamidoamine trees and gold nanoparticles, which is applied in the field of targeted gene transfection of folic acid-functional polyamidoamine dendrimer carriers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
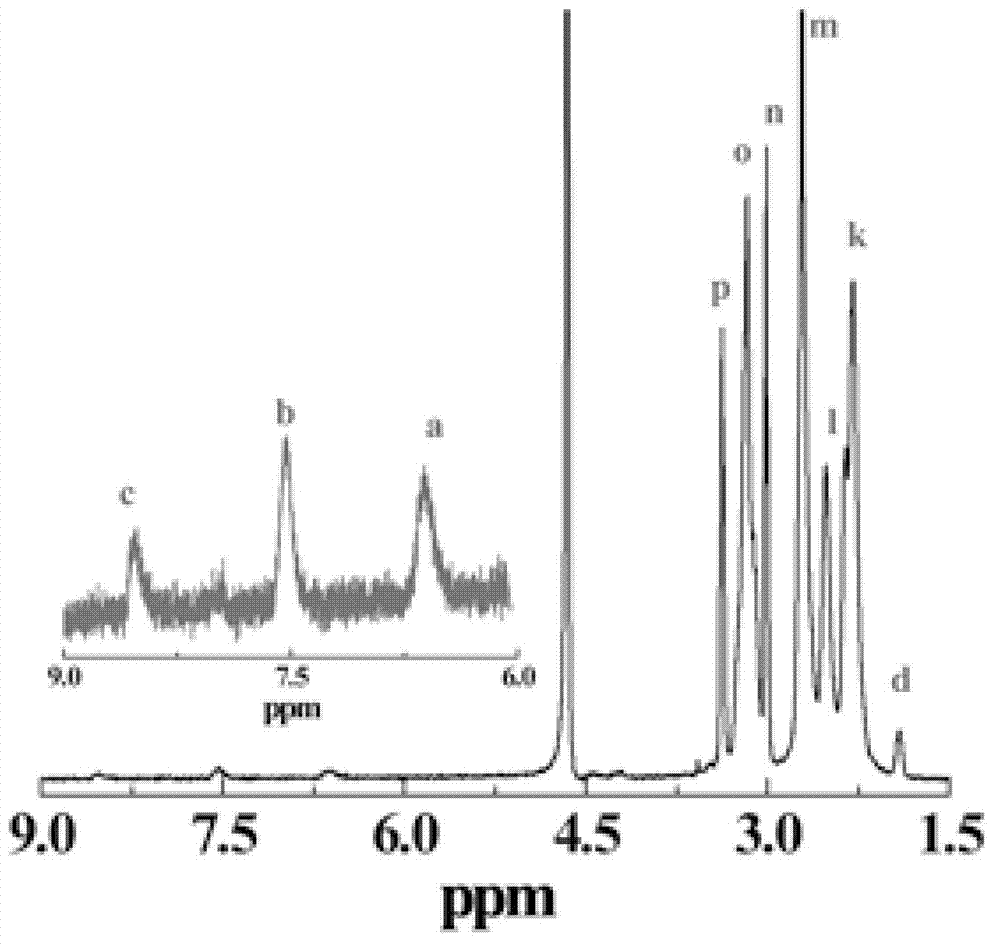
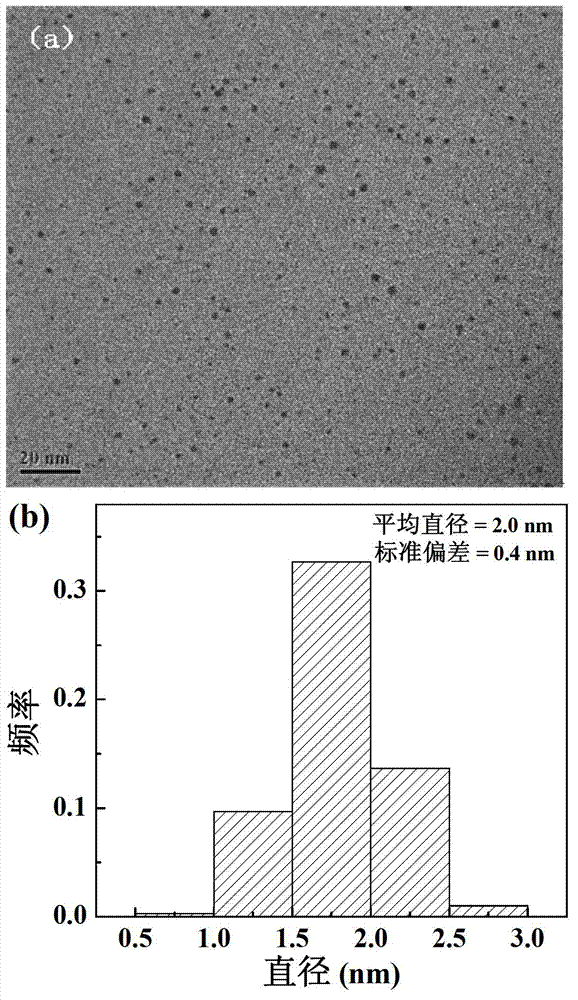
[0053] Weigh 4.22 mg of folic acid (FA) and dissolve it in 3 mL of DMSO. Weigh 18.33mg EDC and dissolve it in 2ml DMSO, add the EDC solution to the FA solution, and stir for 3h. Weigh the fifth generation polyamidoamine dendrimer (G5.NH 2 ) 40mg, dissolved in 4mL DMSO to prepare a solution with a concentration of 10.0mg / mL. According to FA / G5.NH 2 The molar ratio is 5 / 1, add the activated folic acid solution in (1) to G5.NH 2 Solution, magnetic stirring at room temperature, reaction 3d. Add 0.42mL HAuCl to the above solution 4 The aqueous solution (20mg / mL) was mixed and stirred for half an hour, and then 0.453mL of 10mg / mL NaBH was added 4 Solution (H 2 O:CH 3 OH (volume ratio)=2:1), react for 2h. After the reaction, the reaction product was transferred to a dialysis bag with a molecular weight cut-off of 14,000, and dialyzed in distilled water for two days (2 L×6, three times a day). Then freeze-drying was carried out to obtain the Au DENPs-FA reaction product. The...
Embodiment 2
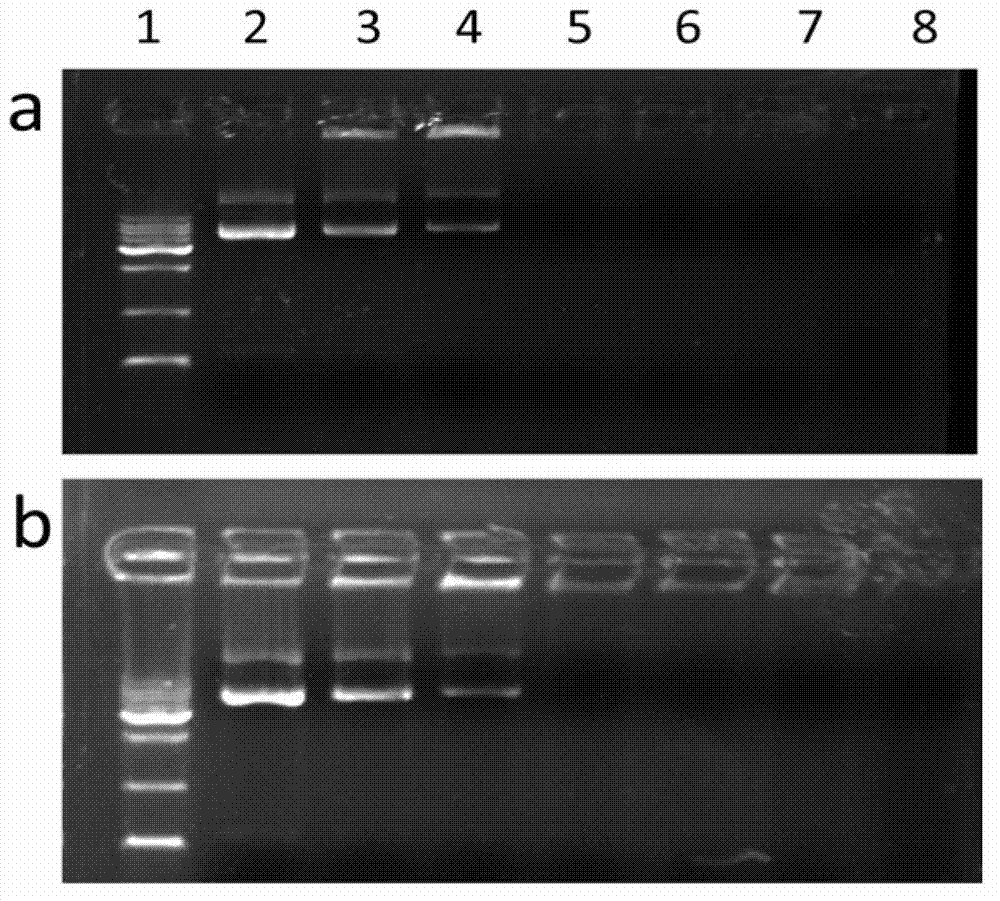
[0055] The Au DENPs-FA prepared according to the method of Example 1 and the Au DENPs prepared in Comparative Example 1 formed complexes with pDNA, and a gel retardation experiment was performed. Prepare 8 wells of agarose gel (1.0% w / v) containing ethidium bromide (0.1 μg / mL), and place at room temperature until the agarose gel solidifies. Taking 1 μg of pDNA as an example, prepare vector / pDNA complexes according to different N / P (0.125, 0.25, 0.5, 1, 2, 5), and use naked pDNA as a control. Then the corresponding vector / pDNA complexes were respectively added to the wells of the agarose gel with a voltage of 80V and a time of 30min. The migration of DNA in the gel was analyzed using a gel imager. The result is as shown in Figure 3. The results showed that both Au DENPs-FA and Au DENPs could well complex with DNA at a low N / P (N / P=0.5) and block DNA.
Embodiment 3
[0057] After the Au DENPs-FA prepared according to the method of Example 1 and the Au DENPs prepared in Comparative Example 1 were complexed with 5 μg DNA (N / P=1, 2.5, 5), the volume was adjusted to 1 mL with deionized water respectively. The particle size and surface potential were characterized by a Malvern laser particle size analyzer (Malvern, MK, 633nm laser), and the results are shown in Figure 4. The results showed that with the increase of N / P, the sizes of all complexes first decreased and then slightly increased. Under the same N / P, the size of Au DENPs-FA / pDNA complex was not much different from that of Au DENPs / pDNA complex. In addition, with the increase of N / P, the surface potential of Au DENPs-FA / pDNA complexes and Au DENPs / pDNA complexes both increased.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com