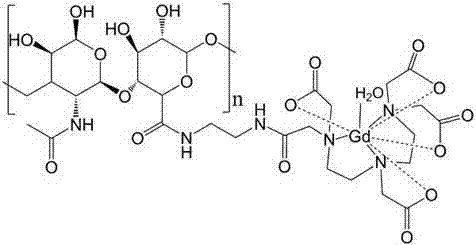
Gadolinium-containing macromolecular contrast agent and preparation method thereof
A technology of contrast agents and macromolecules, applied in the field of nuclear magnetic resonance targeted contrast agents and their preparation, can solve the problems of inability to target and locate contrast imaging, and achieve the effect of improving sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A. Fully dissolve hyaluronic acid HA with a molecular weight of 6,800 in MES buffer solution to prepare 50 mL of a solution with a hyaluronic acid concentration of 0.5 g / L. Slowly add the condensing agent under stirring condition: 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) 200mg, N-hydroxysuccinimide (NHS) 300mg, react 15min, adjust the pH to 7 with PBS, add ethylenediamine, react for 24h, put the reaction solution into the dialysis bag, dialyze in deionized water for 8 days, then freeze-dry to obtain product A, and then dissolve the obtained product in 50mL MES buffer solution to obtain solution A,
[0028] B. Dissolve diethylenetriaminepentaacetic acid in MES buffer solution, prepare 50mL of a solution with a concentration of 0.5g / L, slowly add the condensing agent 1-(3-dimethylaminopropyl)-3-ethyl under stirring condition Carbodiimide hydrochloride 200mg, N-hydroxysuccinimide 300mg, react for 15min, adjust pH7 with PBS, add A solution, react...
Embodiment 2
[0031] A. Fully dissolve hyaluronic acid HA with a molecular weight of 10,000 in MES buffer solution to prepare 50 mL of a solution with a hyaluronic acid concentration of 0.5 g / L. Slowly add the condensing agent under stirring condition: 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) 200mg, N-hydroxysuccinimide (NHS) 300mg, react 15min, adjust the pH to 7 with PBS, add ethylenediamine, react for 24h, put the reaction solution into the dialysis bag, dialyze in deionized water for 8 days, then freeze-dry to obtain product A, and then dissolve the obtained product in 50mL MES buffer solution to obtain solution A,
[0032] B. Dissolve diethylenetriaminepentaacetic acid in MES buffer solution, prepare 50mL of a solution with a concentration of 0.5g / L, slowly add the condensing agent 1-(3-dimethylaminopropyl)-3-ethyl under stirring condition Carbodiimide hydrochloride 200mg, N-hydroxysuccinimide 300mg, react for 15min, adjust pH7 with PBS, add A solution, reac...
Embodiment 3
[0035]A. Fully dissolve hyaluronic acid HA with a molecular weight of 50,000 in MES buffer solution to prepare 50 mL of a solution with a hyaluronic acid concentration of 0.5 g / L. Slowly add the condensing agent under stirring condition: 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) 200mg, N-hydroxysuccinimide (NHS) 300mg, react 15min, adjust the pH to 7 with PBS, add ethylenediamine, react for 24h, put the reaction solution into the dialysis bag, dialyze in deionized water for 8 days, then freeze-dry to obtain product A, and then dissolve the obtained product in 50mL MES buffer solution to obtain solution A,
[0036] B. Dissolve diethylenetriaminepentaacetic acid in MES buffer solution, prepare 50mL of a solution with a concentration of 0.5g / L, slowly add the condensing agent 1-(3-dimethylaminopropyl)-3-ethyl under stirring condition Carbodiimide hydrochloride 200mg, N-hydroxysuccinimide 300mg, react for 15min, adjust pH7 with PBS, add A solution, react...
PUM
| Property | Measurement | Unit |
|---|---|---|
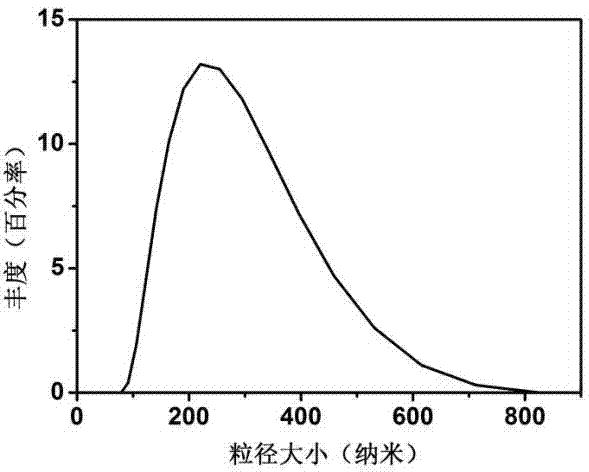
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com