Preparation technology of anthracyclines lipidosome injecta
A preparation process, liposome technology, applied in the field of medicine, can solve the problems of high cost, difficulty in scaling up the production process, difficulty in ensuring production reproducibility and continuity, etc., to expand production scale, facilitate sterility assurance, reduce The effect of human interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
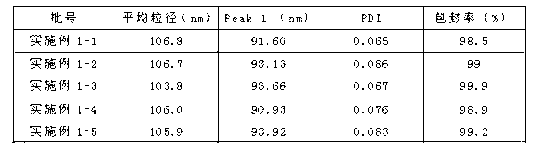
Embodiment 1
[0025] The preparation of daunorubicin hydrochloride liposome injection comprises the following steps:
[0026] (1) Preparation of liposomes by microfluidic focusing technology:
[0027] 1 dissolve 40 mg of hydrogenated soybean lecithin and 10 mg of cholesterol in 10 ml of dehydrated ethanol solution to prepare a solution with a concentration of phospholipids of 4.0 g / L and a concentration of cholesterol of 1.0 g / L as the oil phase;
[0028] II hydrochloric acid, glucose, ammonium sulfate and daunorubicin hydrochloride are dissolved in 200ml water for injection, and the final concentration of hydrochloric acid, glucose, ammonium sulfate is respectively 10mM / L, 55mM / L, 250mM / L, 1.0g / L The solution is used as the water phase, and the pH of the water phase solution is below 4;
[0029] III The water phase prepared above is heated by a heat exchanger under the drive of a peristaltic pump to form a liquid at 30°C, enters the three-way device and forms two equal-volume injection wa...
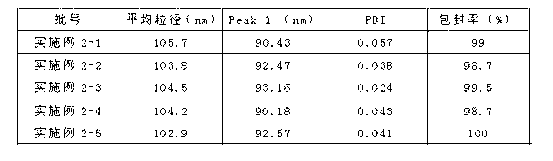
Embodiment 2
[0037] The preparation of doxorubicin hydrochloride liposome injection comprises the following steps:
[0038] (1) Preparation of liposomes by microfluidic focusing technology:
[0039] 1. Hydrogenated soybean lecithin 28mg, cholesterol 10mg are dissolved in 5ml dehydrated ethanol solution and are formulated into the solution that phospholipid concentration is 5.6g / L, cholesterol concentration is 2.0g / L as oil phase;
[0040]II Dissolve hydrochloric acid, maltose, citric acid and doxorubicin hydrochloride solution in 100ml water for injection to prepare the final concentrations of hydrochloric acid, maltose and citric acid to be 10mM / L, 200mM / L, 250mM / L, 1.0g / L respectively The solution is used as the water phase, and the pH of the water phase solution is below 4;
[0041] III The water phase prepared above is heated by a heat exchanger under the drive of a peristaltic pump to form a liquid at 50°C, enters the three-way device and forms two equal-volume injection water flows ...
experiment example 1
[0051] Experimental Example 1 Comparing the Effects of Liposomes Prepared by Different Methods
[0052] Experimental technical route
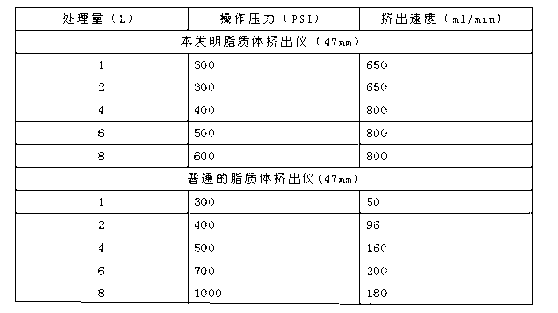
[0053] Get 2g of hydrogenated soybean lecithin, 0.5g of cholesterol, according to film-dissolving dispersion method, manual ethanol injection method, nitrogen as driving force, ethanol injection method, the present invention respectively, and get each sample 1ml and carry out the mensuration of particle diameter. The results are shown in Table 3
[0054] Table 3 Liposome particle size and distribution of different preparation methods
[0055]
[0056] Conclusion: as can be seen from above-mentioned experiment 1, adopt the liposome prepared by the present invention, its particle diameter and PDI are better than first two methods, and avoid and overcome the deficiency of first two methods, be used for liposome Large-scale production is of great significance.
[0057] Whole Part II Liposomes
[0058] This part describes the two methods of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com