Method for extracting lithium salt from lithium brine
A technology for extracting lithium and brine, which is applied in the direction of lithium carbonate;/acid carbonate, lithium halide, etc., can solve the problems of high cost and inability to industrialize, so as to improve utilization rate, avoid hydrolysis, and reduce production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
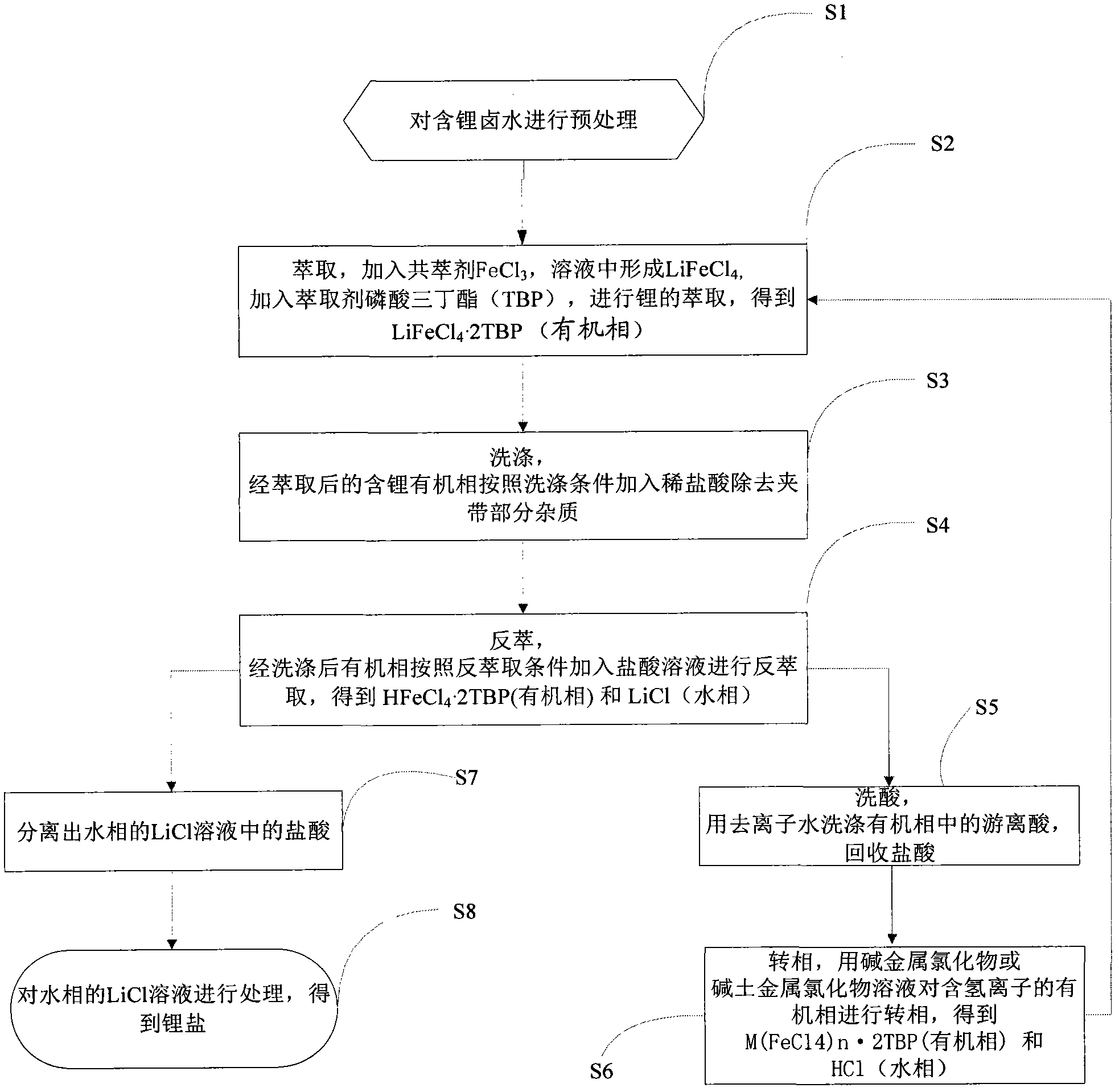
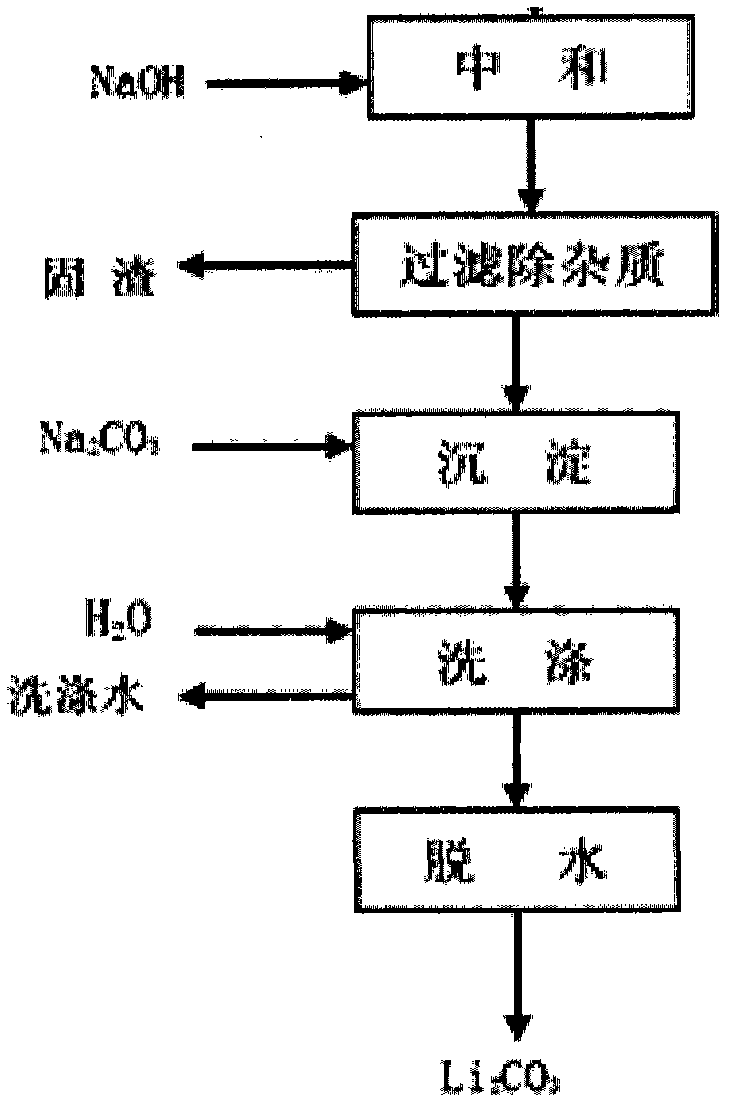
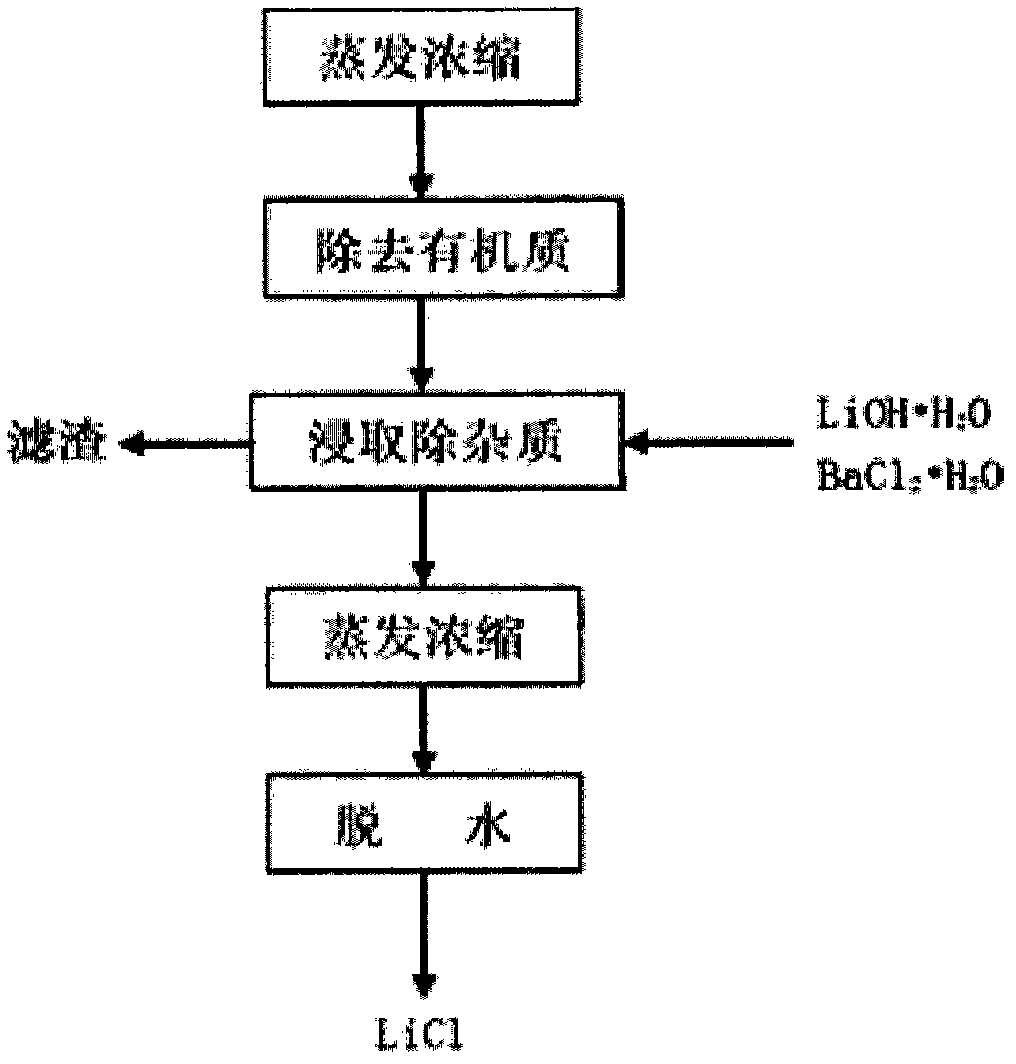
[0048] Add 1 part of a certain salt lake lithium-containing brine (Mg / Li=1~1630 molar ratio, hydrogen ion concentration=0.01~0.10 molar concentration) into a separating funnel, add a certain amount of FeCl 3 (concentration in brine to 0.05-3.0mol / L) as a co-extraction agent, shake to dissolve. Add 1-3 parts of extractant (compared to O / A=1-3), the content of tributyl phosphate extractant in the organic phase is 40-100% (V%), and shake for less than 10 minutes to extract. After the oscillation is over, the static stratification and phase separation. The extraction rate of Li can be greater than 97% through multi-stage countercurrent extraction or fractional distillation extraction process. The extracted lithium-containing organic phase LiFeCl 4 2TBP add dilute hydrochloric acid (H + Concentration=0.05~2.0mol / L)) to remove some impurities entrained. After washing, the organic phase LiFeCl 4 2TBP was added concentrated hydrochloric acid (H + Concentration=2~10 mole mol / L) s...
specific Embodiment 2
[0051] Add 1 part of a certain salt lake lithium-containing brine (Mg / Li=1~1630 molar ratio, hydrogen ion concentration=0.01~0.10 molar concentration) into a separating funnel, add a certain amount of FeCl 3 (concentration in brine to 0.05-3.0mol / L) as a co-extraction agent, shake to dissolve. Add 1-3 parts of extractant (compared to O / A=1-3), the content of tributyl phosphate extractant in the organic phase is 40-100% (V%), and shake for less than 10 minutes to extract. After the oscillation is over, the static stratification and phase separation. The extraction rate of Li can be greater than 97% through multi-stage countercurrent extraction or fractional distillation extraction process. The extracted lithium-containing organic phase LiFeCl 4 2TBP add dilute hydrochloric acid (H + Concentration=0.05~2.0mol / L)) to remove some impurities entrained. After washing, the organic phase LiFeCl 4 2TBP was added concentrated hydrochloric acid (H + Concentration=2~10 mole mol / L) s...
specific Embodiment 3
[0054] Add 1 part of a certain salt lake lithium-containing brine (Mg / Li=1~1630 molar ratio, hydrogen ion concentration=0.01~0.10 molar concentration) into a separating funnel, add a certain amount of FeCl 3(concentration in brine to 0.05-3.0mol / L) as a co-extraction agent, shake to dissolve. Add 1-3 parts of extractant (compared to O / A=1-3), the content of tributyl phosphate extractant in the organic phase is 40-100% (V%), and shake for less than 10 minutes to extract. After the oscillation is over, the static stratification and phase separation. The extraction rate of Li can be greater than 97% through multi-stage countercurrent extraction or fractional distillation extraction process. The extracted lithium-containing organic phase LiFeCl 4 2TBP add dilute hydrochloric acid (H + Concentration=0.05~2.0mol / L)) to remove some impurities entrained. After washing, the organic phase LiFeCl 4 2TBP was added concentrated hydrochloric acid (H + Concentration=2~10 mole mol / L) so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com