Medicine composition, and preparation and application thereof
A technology of pharmaceutical preparations and compositions, applied in the field of pharmaceutical compositions containing indane amide compounds, which can solve the problems of inability to specifically act on cancer cells, hair loss, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
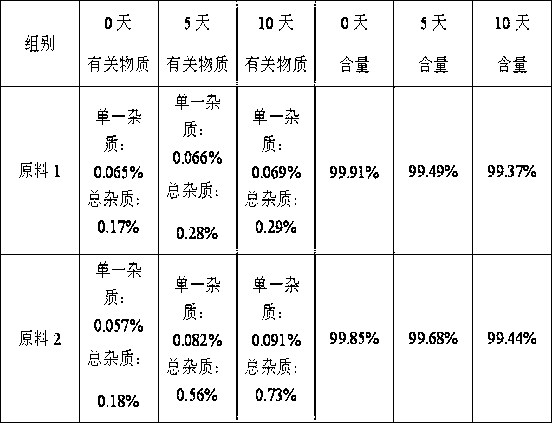
[0065] The effect on prostate cancer PC-3 cells
[0066] 1. Cells and culture conditions: Human prostate cancer PC-3 cells were cultured in RPMI1640 medium containing 10% calf serum, supplemented with 100 U / ml penicillin and 100 mg / L streptomycin. Routine cultivation was carried out at 37°C and 50 ml / L CO2. About 5×106 / ml cells in the exponential growth phase were subcultured and inoculated in 24-well plates, and then cultured for 12 h before use.
[0067] 2. Experimental drugs
[0068] Experimental drug group 1: docetaxel 6.7×10-5g / L;
[0069] Experimental drug group 2: (1S)-1-(4-methylpiperazin-1-yl)-N-(4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino ]Phenyl)-2,3-indane-5-amide sesquisulfate 6.7×10-5g / L;
[0070] Experimental drug group 3: (1S)-1-(4-methylpiperazin-1-yl)-N-(4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino ]phenyl)-2,3-indane-5-position amide sesquisulfate 6.7×10-5g / L; docetaxel 6.7×10-5g / L;
[0071] Experimental drug group 4: (1S)-1-(4-methylpiperazin-1...
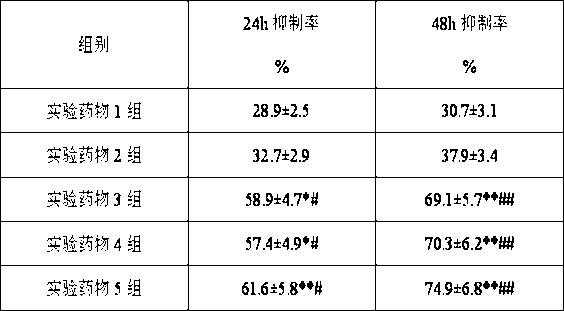
experiment example 2
[0079] Study on antitumor effect in human chronic myeloid leukemia K562 tumor model
[0080] 1. Experimental animals: NOD / SCID mice, female, 6-8 weeks old, weighing 18-22 g.
[0081] 2. Experimental drugs:
[0082] Experimental drug group 1: (1S)-1-(4-methylpiperazin-1-yl)-N-(4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino ]Phenyl)-2,3-indane-5-amide sesquisulfate 90mg / kg.
[0083] Experimental drug group 2: docetaxel 10 mg / kg.
[0084] Experimental drug group 3: (1S)-1-(4-methylpiperazin-1-yl)-N-(4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino ]Phenyl)-2,3-indane-5-amide sesquisulfate 90mg / kg, docetaxel 10mg / kg.
[0085] Experimental drug group 4: (1S)-1-(4-methylpiperazin-1-yl)-N-(4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino ]Phenyl)-2,3-indane-5-amide sesquisulfate 40mg / kg, docetaxel 10mg / kg.
[0086] Experimental drug group 5: (1S)-1-(4-methylpiperazin-1-yl)-N-(4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino ]Phenyl)-2,3-indane-5-amide sesquisulfate 25mg / kg, doce...
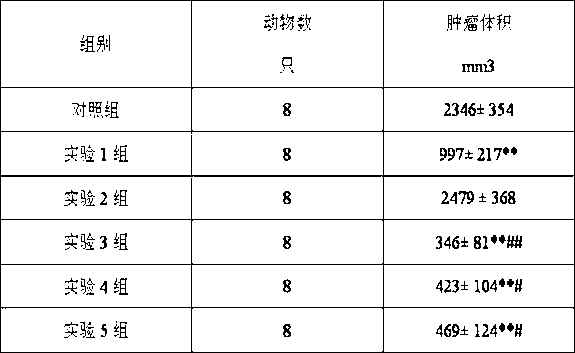
experiment example 3
[0097] Effects on lung cancer in mice
[0098] 1. Experimental animals: C57BL / 6 pure line mice, female, 6-8 weeks old, weighing 18-22 g.
[0099] 2. Tumor strain: Lewis lung cancer cell line (3LL), preserved in C57BL / 6 mice, passaged once every 2 weeks.
[0100] 3. Experimental drugs:
[0101] Experimental drug group 1: (1S)-1-(4-methylpiperazin-1-yl)-N-(4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino ]Phenyl)-2,3-indane-5-amide sesquisulfate 20mg / kg.
[0102] Experimental drug group 2: cephalomannine 20 mg / kg.
[0103] Experimental drug group 3: (1S)-1-(4-methylpiperazin-1-yl)-N-(4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino ]Phenyl)-2,3-indane-5-amide sesquisulfate 18mg / kg, cephalomannine 2mg / kg.
[0104] Experimental drug group 4: (1S)-1-(4-methylpiperazin-1-yl)-N-(4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino ]Phenyl)-2,3-indane-5-amide sesquisulfate 15mg / kg, cephalomannine 5mg / kg.
[0105] Experimental drug group 5: (1S)-1-(4-methylpiperazin-1-yl)-N-(4-methyl-3-[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com