CO2 absorbent and preparation method thereof
An absorbent and a certain amount of technology, applied in the field of CO2 absorbent and its preparation, can solve the problem of low metal oxide capture performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
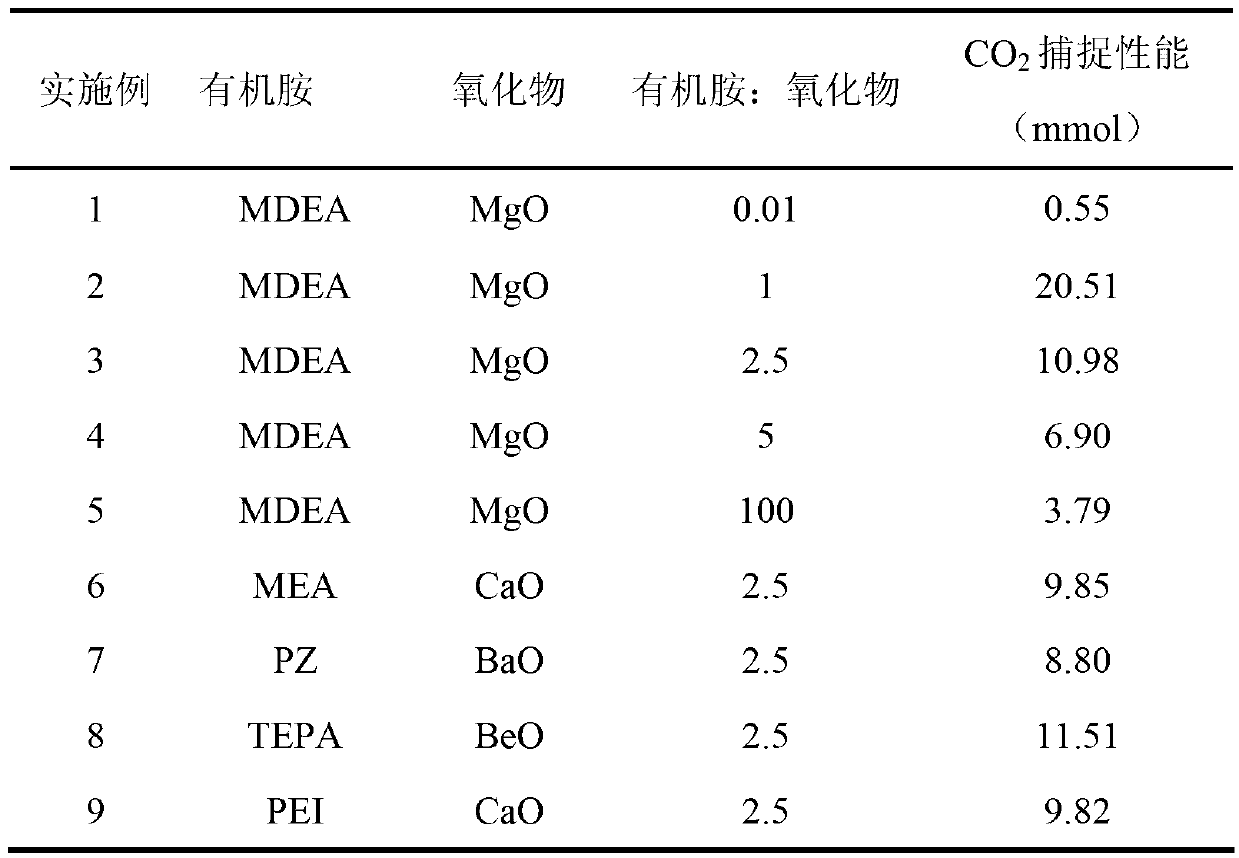
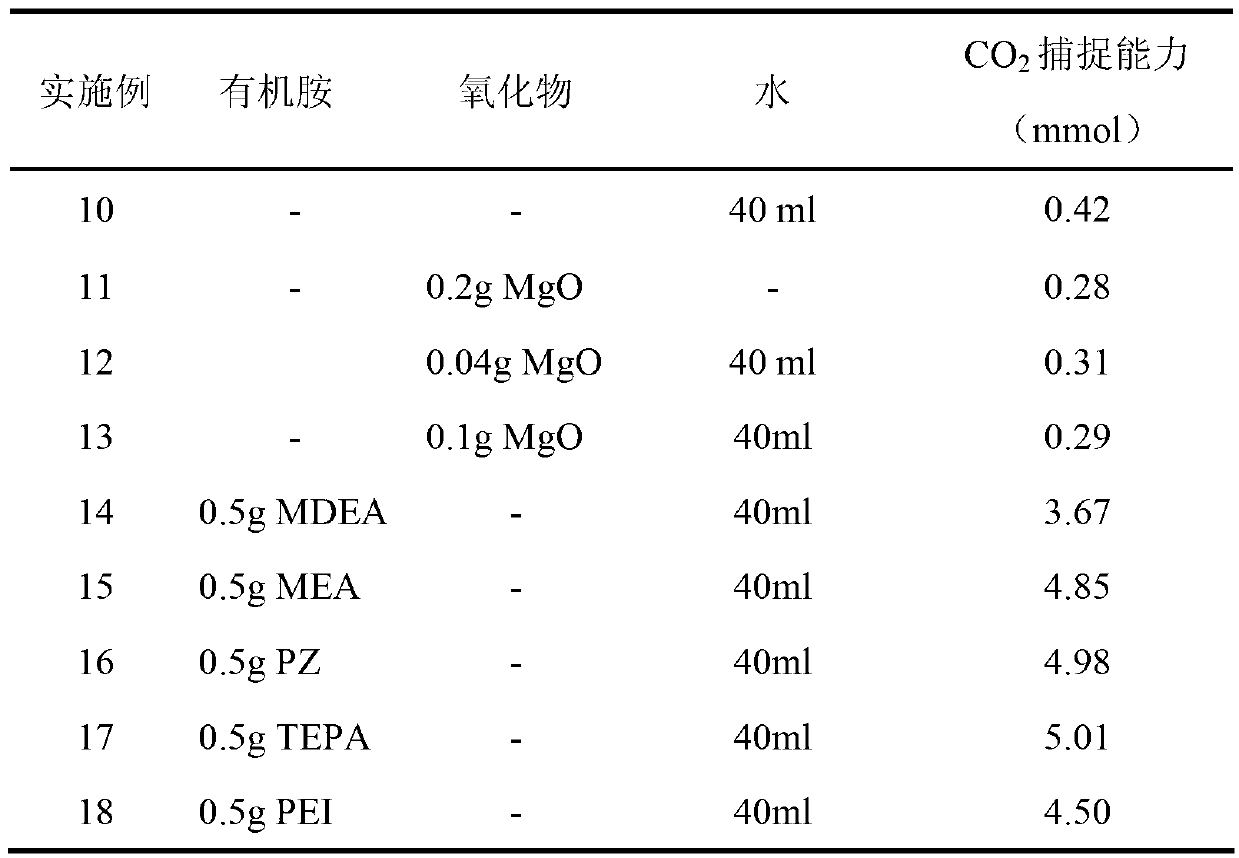
[0015] Preparation of organic amine-metal oxide composite absorbent: Dissolve 0.05 g of organic amine N-methyldiethanolamine (MDEA) in 400 ml of water, then add 5 g of basic metal oxide magnesium oxide, ultrasonicate for 10 minutes, and evenly dissolve magnesium oxide Suspended and dispersed in MDEA aqueous solution to prepare MDEA-MgO composite absorbent.
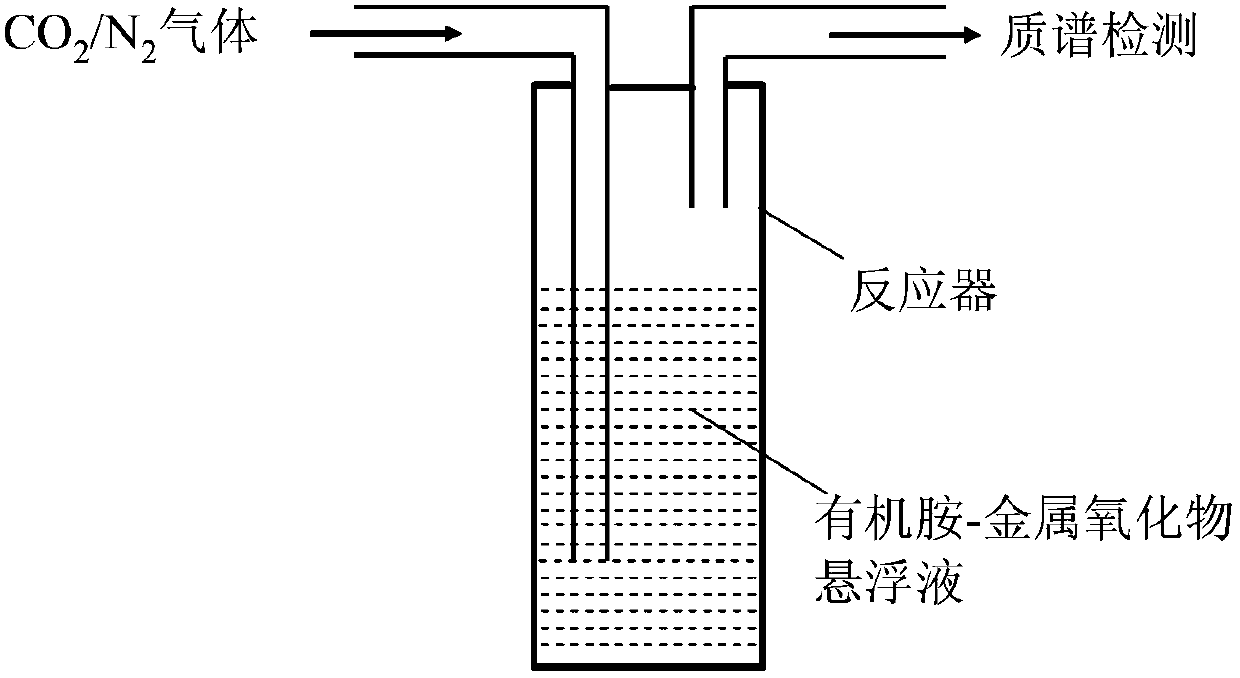
[0016] In a 50ml glass reactor, the CO 2 For the capture capacity test, 40.5ml of the above-mentioned composite absorbent is loaded into a glass reactor, and the absorption temperature is controlled at 25°C. CO was introduced into the reactor 2 and N 2 , CO 2 The flow rate is 5ml / min, N 2 The flow rate is 45ml / min. CO 2 and N 2 After mixing, it enters the reactor, and the gas reaction product is analyzed by mass spectrometry after being absorbed by the absorbent. Absorbent captures CO 2 The capabilities can be seen in Table 1. The flow chart of the reaction device is attached figure 1 .
Embodiment 2
[0018] Preparation of organic amine-metal oxide composite absorbent: Dissolve 5 g of organic amine N-methyldiethanolamine (MDEA) in 400 ml of water, then add 5 g of basic metal oxide magnesium oxide, and ultrasonicate for 10 minutes to uniformly dissolve magnesium oxide Suspended and dispersed in MDEA aqueous solution to prepare MDEA-MgO composite absorbent.
[0019] In a 50ml glass reactor, the CO 2 For the capture capacity test, 40.5ml of the above-mentioned composite absorbent is loaded into a glass reactor, and the absorption temperature is controlled at 25°C. CO was introduced into the reactor 2 and N 2 , CO 2 The flow rate is 5ml / min, N 2 The flow rate is 45ml / min. CO 2 and N 2 After mixing, it enters the reactor, and the gas reaction product is analyzed by mass spectrometry after being absorbed by the absorbent. Absorbent captures CO 2 The capabilities can be seen in Table 1. The flow chart of the reaction device is attached figure 1 .
Embodiment 3
[0021] Preparation of organic amine-metal oxide composite absorbent: Dissolve 5 g of organic amine N-methyldiethanolamine (MDEA) in 400 ml of water, then add 2 g of basic metal oxide magnesium oxide, stir for 10 minutes, and dissolve magnesium oxide evenly Suspended and dispersed in MDEA aqueous solution to prepare MDEA-MgO composite absorbent.
[0022] In a 50ml glass reactor, the CO 2 For the capture capacity test, 40.5ml of the above-mentioned composite absorbent is loaded into a glass reactor, and the absorption temperature is controlled at 25°C. CO was introduced into the reactor 2 and N 2 , CO 2 The flow rate is 5ml / min, N 2 The flow rate is 45ml / min. CO 2 and N 2 After mixing, it enters the reactor, and the gas reaction product is analyzed by mass spectrometry after being absorbed by the absorbent. Absorbent captures CO 2 The capabilities can be seen in Table 1. The flow chart of the reaction device is attached figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com