Method for synthesizing ester by using cutinase
A cutinase and reaction system technology, applied in the field of enzyme engineering, can solve the problems of high enzyme addition and low conversion rate of long-chain ester synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
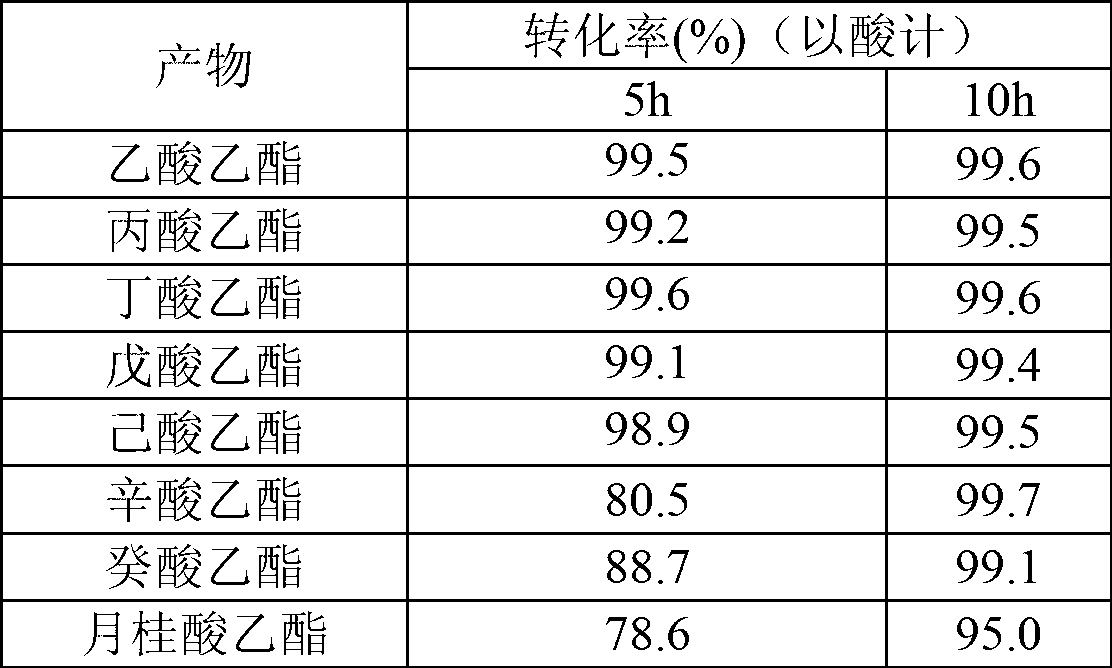
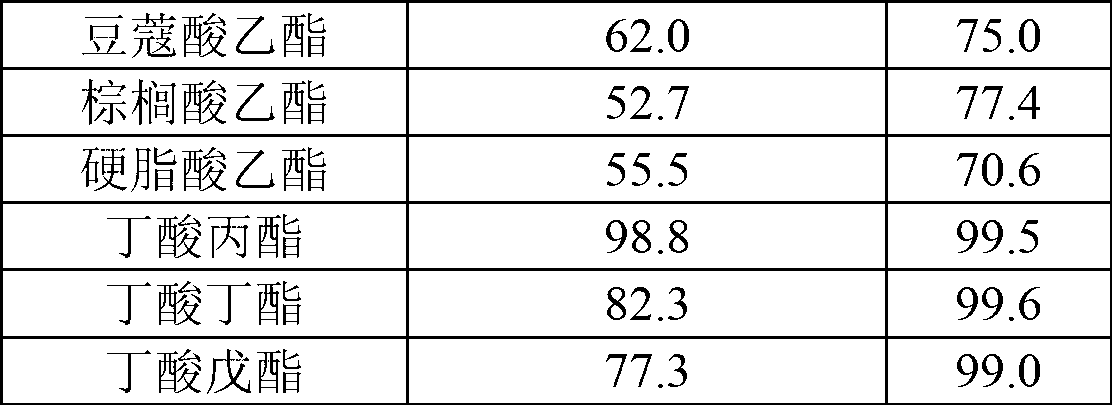
[0015] In this example, the cutinase Tfu_0883 derived from Thermobifida fusca was used to catalyze the reaction.
[0016] Organic reagents and substrate acids and alcohols are used for ester synthesis after removing water with 4A molecular sieve. Reaction conditions: 0.1M acid, 0.2M alcohol, 0.03% water added, 50°C, 150rpm, enzyme 30U / ml, reaction solvent isooctane, the specific results are shown in Table 1 and Table 2.
[0017] Table 1
[0018]
[0019]
[0020] Table 2
[0021]
Embodiment 2
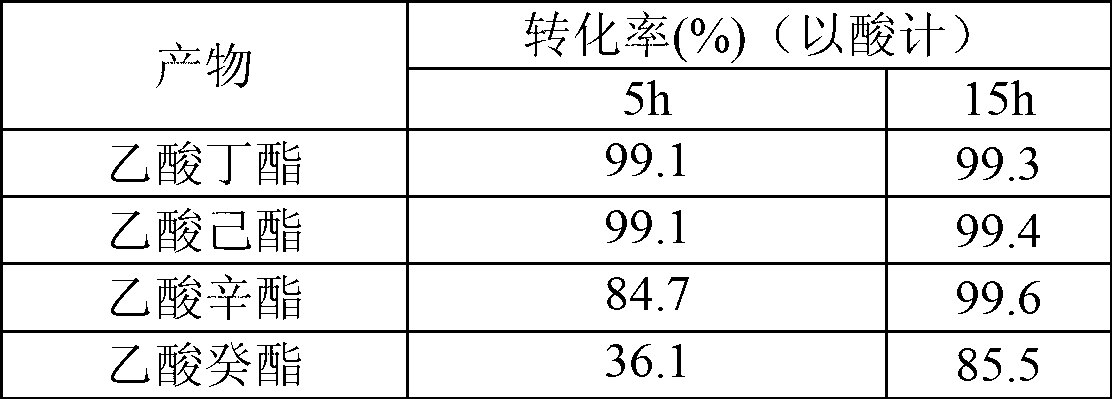
[0023] In this example, the cutinase Tfu_0882 derived from Thermobifida fusca was used to catalyze the reaction.
[0024] Reaction conditions: 0.15M acid, 0.3M alcohol, 0.05% water addition, 50°C, 150rpm, enzyme 40U / ml, reaction solvent isooctane, the specific results are shown in Table 3 and Table 4.
[0025] table 3
[0026]
[0027] Table 4
[0028]
Embodiment 3
[0030] In this example, the cutinase Tfu_0883 derived from Thermobifda fusca was used to catalyze the reaction.
[0031] Reaction conditions: 0.5M acid, 0.5M alcohol, 0.08% water added, 50°C, 150rpm, enzyme 60U / ml, reaction solvent isooctane. After reacting for 5h and 15h, the conversion rates of propyl butyrate were 92.8% and 91.5%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com