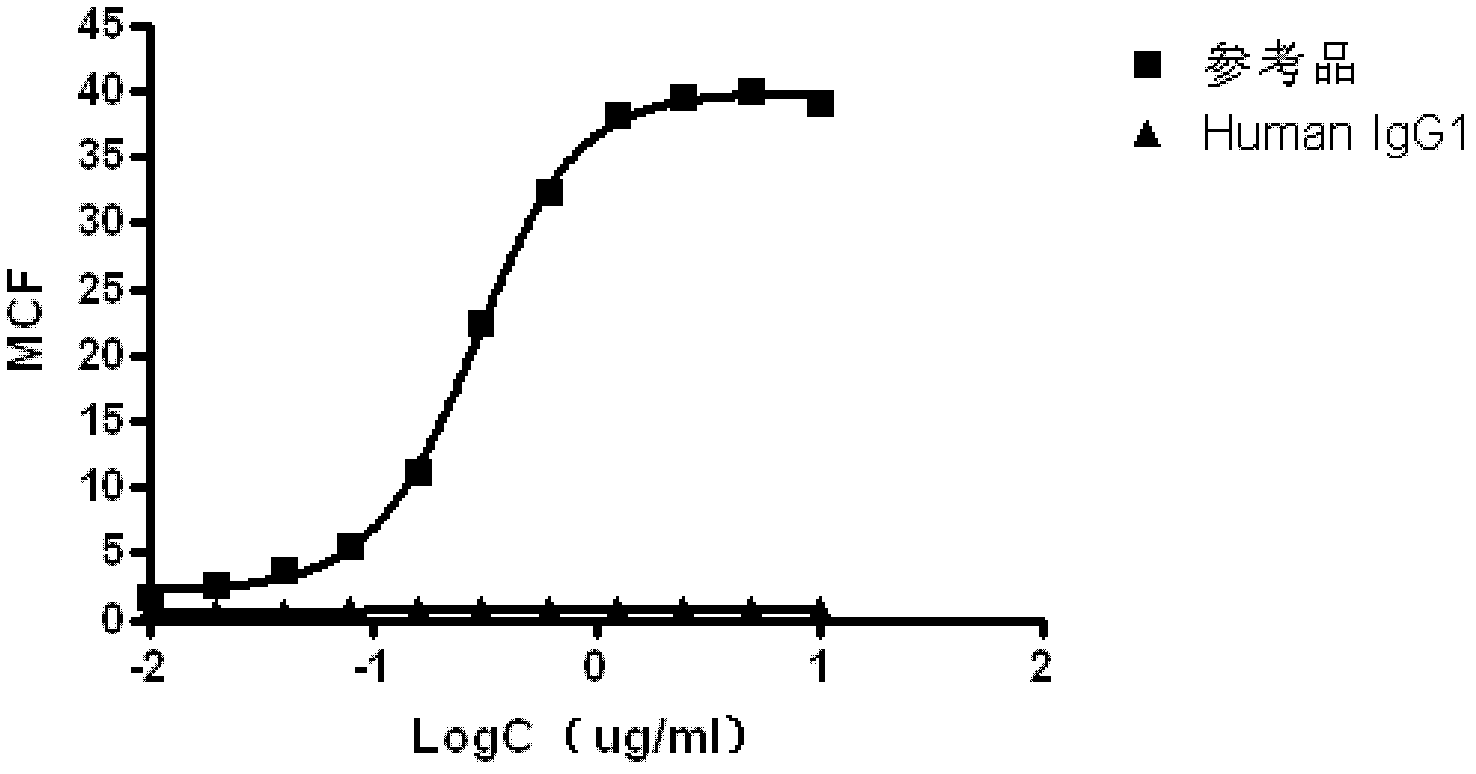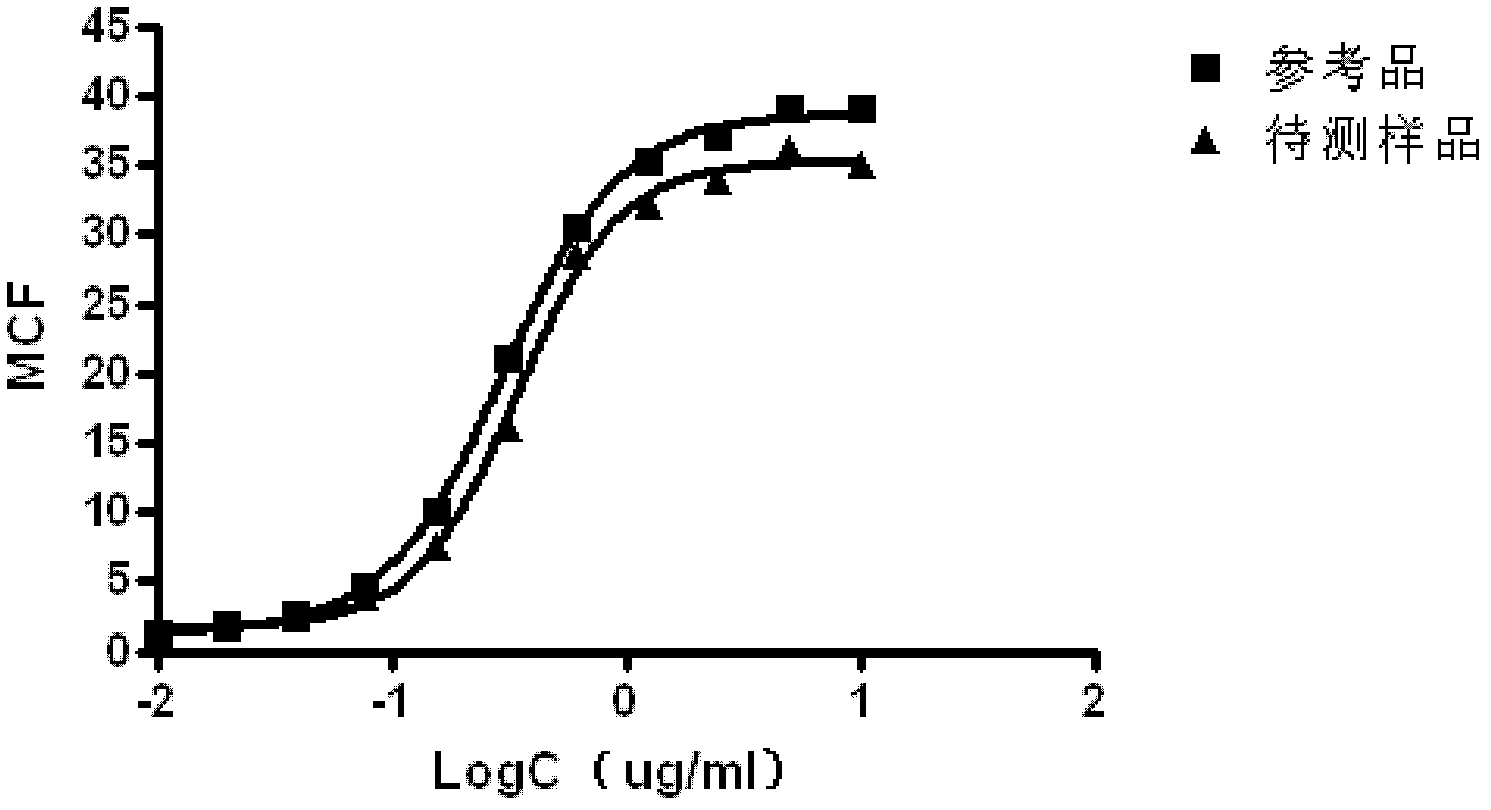Detection method for anti-CD20 monoclonal antibody binding activities
A monoclonal antibody and detection method technology, applied in the field of biomedicine, can solve the problems of lack of methods, different methods, unstandardized methods, etc., and achieve the effects of high precision, good specificity and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Anti-CD20 Monoclonal Antibody Binding Activity Detection
[0045] Using the anti-CD20 monoclonal antibody test sample (prepared by Jiahe Bio-Pharmaceutical Co., Ltd.) as the material, the above-mentioned method was used to detect its binding activity.
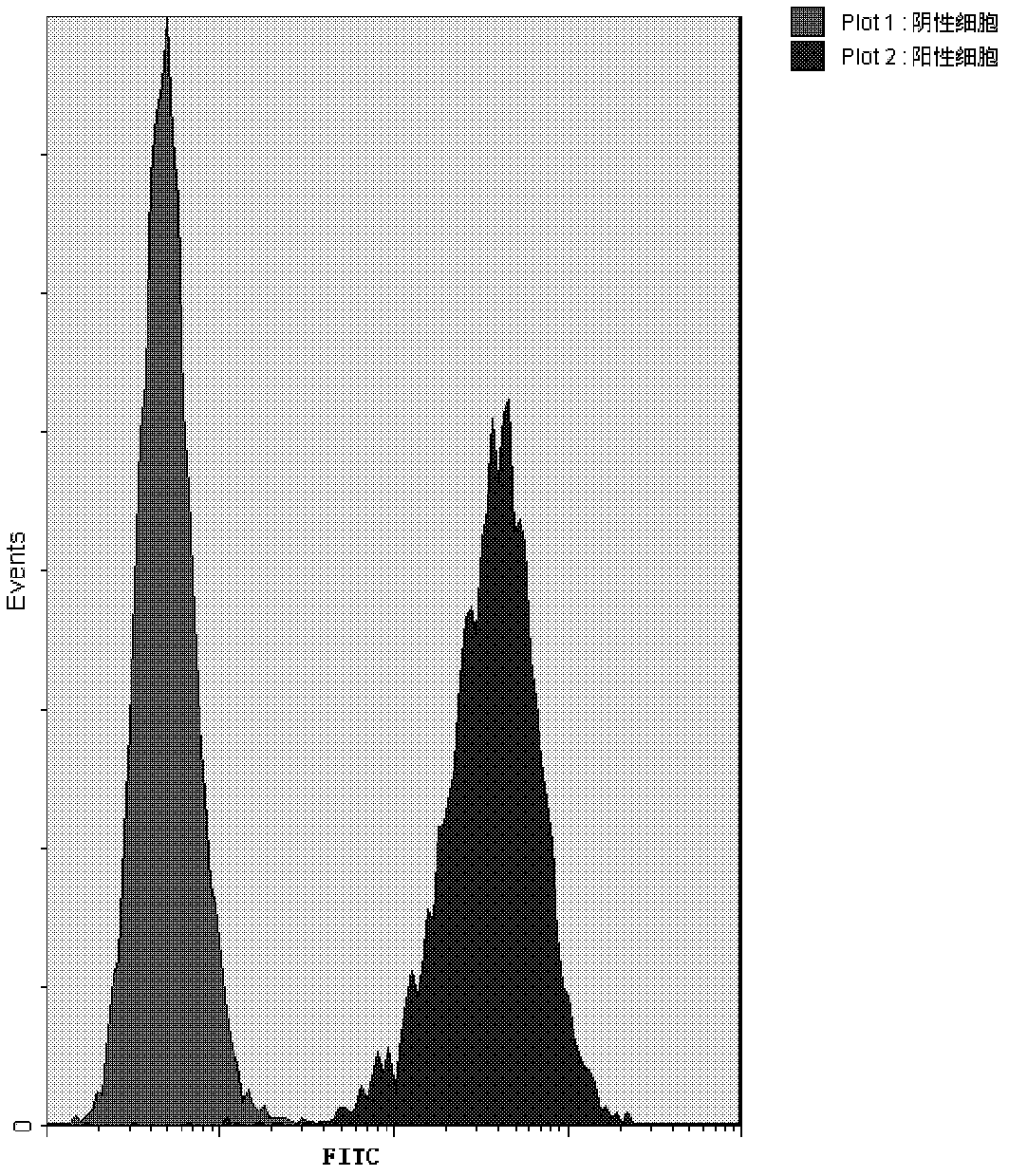
[0046] The result is as figure 1 , the anti-CD20 monoclonal antibody that specifically binds to CD20 on the surface of Raji cells can combine with FITC Mouse anti-Human IgG to generate fluorescence, and the fluorescence intensity increases with the increase of the concentration of the bound anti-CD20 monoclonal antibody, FITC The higher the fluorescence intensity, the peak shape gradually shifted to the right; while the negative control cells did not bind to the anti-CD20 monoclonal antibody, so the position of the peak shape remained unchanged, no right shift occurred, and the fluorescence intensity was extremely low.
[0047] Such as image 3 , the curve fitting of the reference product and the sample to be...
Embodiment 2
[0048] Example 2 Detection of Anti-CD20 Monoclonal Antibody Binding Activity (Specificity Evaluation)
[0049] Human IgG1: Remicade (product of Johnson&Johnson Co.), which does not target the CD20 target, was used as the sample to be tested, and its binding activity was detected by the above-mentioned method.
[0050] The result is as figure 2 , the monoclonal antibody that does not target CD20 does not bind to cells with high expression of CD20 even at the same dilution concentration as the reference product, and the average fluorescence intensity is almost equal to that of the negative control. It can be seen that the detection method of the present invention can specifically detect the binding activity of the monoclonal antibody against the CD20 target.
Embodiment 3
[0051] Example 3 Detection of Anti-CD20 Monoclonal Antibody Binding Activity (Precision Evaluation)
[0052] The precision of the method was evaluated with anti-CD20 monoclonal antibody drug sample (prepared by Jiahe Biopharmaceutical Co., Ltd.) as the material. First, 1 experimenter uses the following method to conduct parallel experiments on the same sample to be tested 5 times to investigate the repeatability of the method; then 2 experimenters use the following method to detect the binding activity of the same sample to be tested, and the personnel who investigate the method Error; Finally, 1 experimenter used the following method to detect the binding activity of the same sample to be tested within 3 different working days, and investigated the inter-day difference of the method.
[0053] The results are shown in Table 1. One experimenter repeated the experiment 5 times on the same sample to be tested, and the RSD of the test result was 2 >0.95 (the curve fitting diagram ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com