Preparation method of high-purity sodium ozagrel
A high-purity technology for sodium ozagrel, which is applied in the field of medicine, can solve the problems that the clarity cannot meet the requirements, cannot solve the problem of clarity, and is infeasible, and achieves the effects of good clarity, simple equipment requirements and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A kind of preparation method of high-purity ozagrel sodium of the present invention is;
[0022] (1) Add 100 grams of ozagrel into 500ml of 10% sodium hydroxide solution, stir to dissolve and clarify.
[0023] (2) The solution is filtered with a 20mm thick Bailu A activated carbon layer;
[0024] (3) Concentrate the filtrate under reduced pressure at 65°C, and stop concentrating when a large number of crystals appear;
[0025] (4) Suction filter the concentrate, wash the obtained solid twice with methanol, and then filter the solid again.
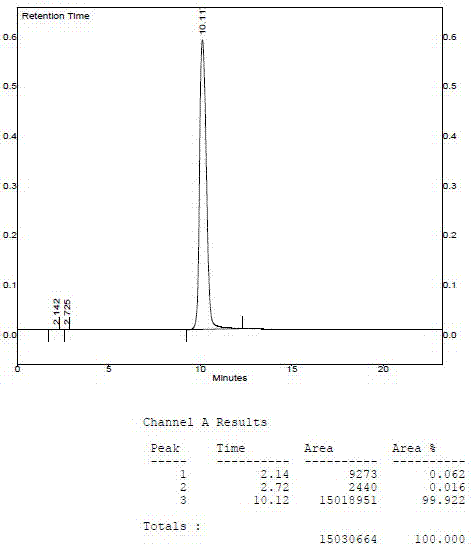
[0026] (5) The obtained solid was dried under reduced pressure at 50°C for 6 hours to obtain 37 grams of sodium ozagrel that complied with the JP16 standard. The purity of ozagrel sodium is over 99.9%, and the clarity is far less than 0.5.
Embodiment 2
[0028] A kind of preparation method of high-purity ozagrel sodium of the present invention is;
[0029] (1) Add 150 grams of ozagrel into 700ml of 15% sodium hydroxide solution, stir to dissolve and clarify.
[0030] (2) The solution is filtered with a 20mm thick Bailu A activated carbon layer;
[0031] (3) Concentrate the filtrate under reduced pressure at 70°C, and stop concentrating when a large number of crystals appear;
[0032] (4) Suction filter the concentrate, wash the obtained solid twice with ethanol, and then filter the solid again.
[0033] (5) The obtained solid was dried under reduced pressure at 60° C. for 8 hours to obtain 57 grams of sodium ozagrel that complied with the JP16 standard. The purity of ozagrel sodium is over 99.9%, and the clarity is far less than 0.5.
Embodiment 3
[0035] A kind of preparation method of high-purity ozagrel sodium of the present invention is;
[0036] (1) Add 200 grams of ozagrel into 500ml of 20% sodium hydroxide solution, stir to dissolve and clarify.
[0037] (2) The solution is filtered with a 20mm-thick Bailu Z activated carbon layer;
[0038] (3) Concentrate the filtrate under reduced pressure at 80°C, and stop concentrating when a large number of crystals appear;
[0039] (4) Suction filter the concentrate, wash the obtained solid twice with ethanol, and then filter the solid again.
[0040] (5) The obtained solid was dried under reduced pressure at 55° C. for 8 hours to obtain 78 grams of sodium ozagrel conforming to the JP16 standard. The purity of ozagrel sodium is over 99.9%, and the clarity is far less than 0.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com