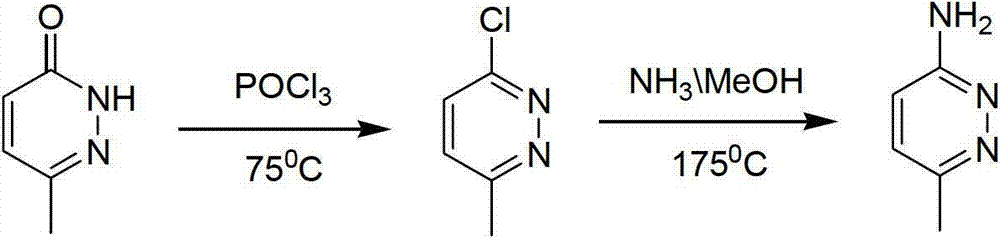
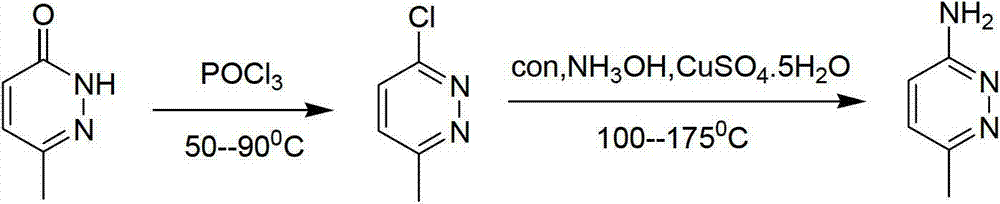
Method for industrial preparation of 6-methyl-3-aminopyridazine
An aminopyridazine and methyl technology, applied in the field of industrialized preparation of 6-methyl-3-aminopyridazine, can solve the problem that there is no effective method for preparing industrial scaled production, raw materials are not well recycled, and the operation reaction process Complexity and other problems, to achieve the effect of large-scale industrial production prospects, effective synthesis yield improvement, simple feeding and post-processing operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The invention relates to an industrial preparation method of 6-methyl-3-aminopyridazine, which uses 6-methyl-3-chloropyridazine as a raw material, and carries out ammoniation reaction with concentrated ammonia water, and obtains the product after water recrystallization and purification 6-Methyl-3-aminopyridazine and by-product 6-methyl-pyridazin-3-one.
[0022] The mass concentration of the concentrated ammonia water is 25%-28%, the temperature of the ammoniation reaction is 120°C-150°C, and the reaction pressure is 0.2-0.4MPa.
[0023] After the ammoniation reaction is completed, after the reaction solution is cooled to room temperature, the pH of the reaction solution is adjusted to 7-8 with dilute hydrochloric acid, and then after standing for 48 hours, a light yellow solid precipitates from the reaction solution, which is filtered to obtain 6-methyl- 3-aminopyridazine.
[0024] Concentrate the filtrate from which 6-methyl-3-aminopyridazine has been filtered out, a...
Embodiment 1
[0028] At room temperature, add 3-chloro-6-methylpyridazine (50g, 0.39mol) and concentrated ammonia water (250ml) into the autoclave, raise the temperature to 120-130 degrees, and the pressure to 0.2-0.3MPa, 120-130 Insulated and stirred for 5-12 hours under the temperature. After cooling down and depressurizing and deflation, test. Adjust the pH to 7 with dilute hydrochloric acid, and let it stand for 48 hours. A large amount of light yellow solid precipitated, and the product 6-methyl-3-aminopyridazine (25.5 g, 0.23 mol) was obtained by filtration, with a purity of 98% and a yield of 60%.
[0029] The above-mentioned filtrate was concentrated to remove two-thirds of the volume of water, the pH was adjusted to 5, and it was left to stand for 24 hours. Off-white solids were precipitated, and the by-product 6-methyl-pyridazin-3-one (15.3g, 0.14mol) was obtained by filtration. 96%.
Embodiment 2
[0031] At room temperature, add 3-chloro-6-methylpyridazine (500g, 3.9mol) and concentrated ammonia water (2.5L) into the autoclave, raise the temperature to 120-130 degrees, and the pressure to 0.2-0.3MPa, 120- Heat preservation and stirring at 130 degrees for 5-12 hours. After cooling down and depressurizing and deflation, test. Adjust the pH to 7 with dilute hydrochloric acid and let it stand for 48 hours. A large amount of light yellow solid precipitated out. The product 6-methyl-3-aminopyridazine (267.7 g, 2.4 mol) was obtained by filtration, with a purity of 98% and a yield of 63%.
[0032] The above-mentioned filtrate was concentrated to remove two-thirds of the volume of water, the pH was adjusted to 5, and it was left to stand for 24 hours. Off-white solids were precipitated, and the by-product 6-methyl-pyridazin-3-one (140.5g, 1.3mol) was obtained by filtration. The purity 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com