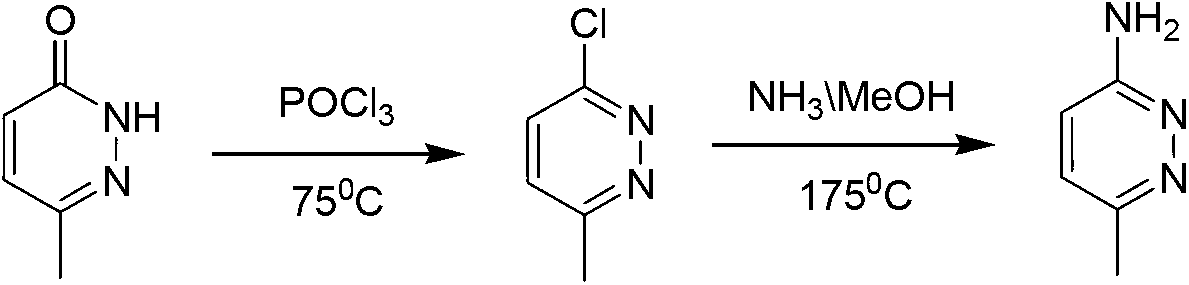
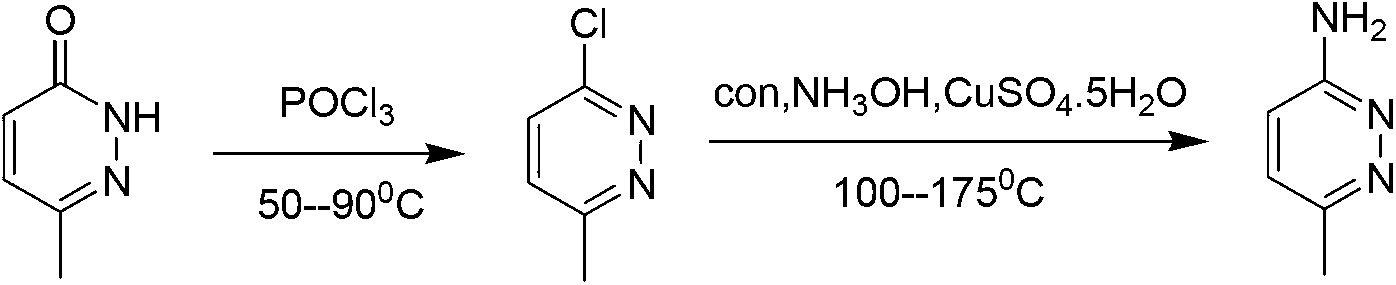
Method for industrial preparation of 6-methyl-3-aminopyridazine
A technology of aminopyridazine and methyl, which is applied in the field of industrialized preparation of 6-methyl-3-aminopyridazine, can solve the problem of no effective preparation method for industrialized scale-up production, poor recycling of raw materials, and operation reaction process Complexity and other issues, to achieve the prospect of large-scale industrial formation, increase the effective synthesis yield, and simple feeding and post-processing operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The invention relates to an industrial preparation method of 6-methyl-3-aminopyridazine, which uses 6-methyl-3-chloropyridazine as a raw material, and carries out ammoniation reaction with concentrated ammonia water, and obtains the product after water recrystallization and purification 6-Methyl-3-aminopyridazine and by-product 6-methyl-pyridazin-3-one.
[0022] The mass concentration of the concentrated ammonia water is 25%-28%, the temperature of the ammoniation reaction is 120°C-150°C, and the reaction pressure is 0.2-0.4MPa.
[0023] After the ammoniation reaction is completed, after the reaction solution is cooled to room temperature, the pH of the reaction solution is adjusted to 7-8 with dilute hydrochloric acid, and then after standing for 48 hours, a light yellow solid precipitates from the reaction solution, which is filtered to obtain 6-methyl- 3-aminopyridazine.
[0024] Concentrate the filtrate from which 6-methyl-3-aminopyridazine has been filtered out, a...
Embodiment 1
[0028] At room temperature, add 3-chloro-6-methylpyridazine (50g, 0.39mol) and concentrated ammonia water (250ml) into the autoclave, raise the temperature to 120-130 degrees, and the pressure to 0.2-0.3MPa, 120-130 Insulated and stirred for 5-12 hours under the temperature. After cooling down and depressurizing and deflation, test. Adjust the pH to 7 with dilute hydrochloric acid, and let it stand for 48 hours. A large amount of light yellow solid precipitated, and the product 6-methyl-3-aminopyridazine (25.5 g, 0.23 mol) was obtained by filtration, with a purity of 98% and a yield of 60%.
[0029] The above-mentioned filtrate was concentrated to remove two-thirds of the volume of water, the pH was adjusted to 5, and it was left to stand for 24 hours. Off-white solids were precipitated, and the by-product 6-methyl-pyridazin-3-one (15.3g, 0.14mol) was obtained by filtration. 96%.
Embodiment 2
[0031] At room temperature, add 3-chloro-6-methylpyridazine (500g, 3.9mol) and concentrated ammonia water (2.5L) into the autoclave, raise the temperature to 120-130 degrees, and the pressure to 0.2-0.3MPa, 120- Heat preservation and stirring at 130 degrees for 5-12 hours. After cooling down and depressurizing and deflation, test. Adjust the pH to 7 with dilute hydrochloric acid and let it stand for 48 hours. A large amount of light yellow solid precipitated out. The product 6-methyl-3-aminopyridazine (267.7 g, 2.4 mol) was obtained by filtration, with a purity of 98% and a yield of 63%.
[0032] The above-mentioned filtrate was concentrated to remove two-thirds of the volume of water, the pH was adjusted to 5, and it was left to stand for 24 hours. Off-white solids were precipitated, and the by-product 6-methyl-pyridazin-3-one (140.5g, 1.3mol) was obtained by filtration. The purity 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com