Production method of photosensitive resin and photosensitive resin composition
A technology of photosensitive resin and manufacturing method, which is applied in the field of photosensitive resin manufacture and photosensitive resin composition, can solve problems such as catalyst residue, substrate metal corrosion, resin hue, acid value change, etc., achieve stable electrical characteristics, simplify Excellent effect of manufacturing process and PCT resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
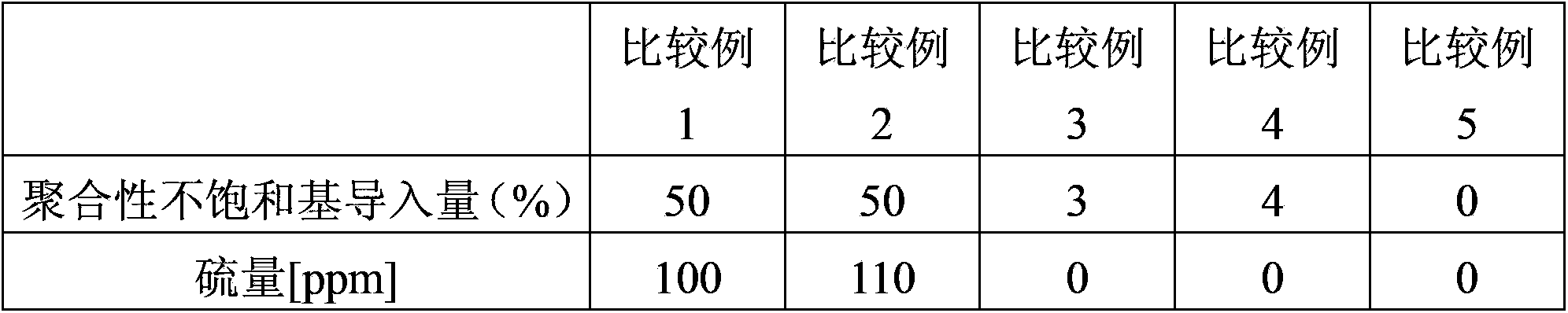
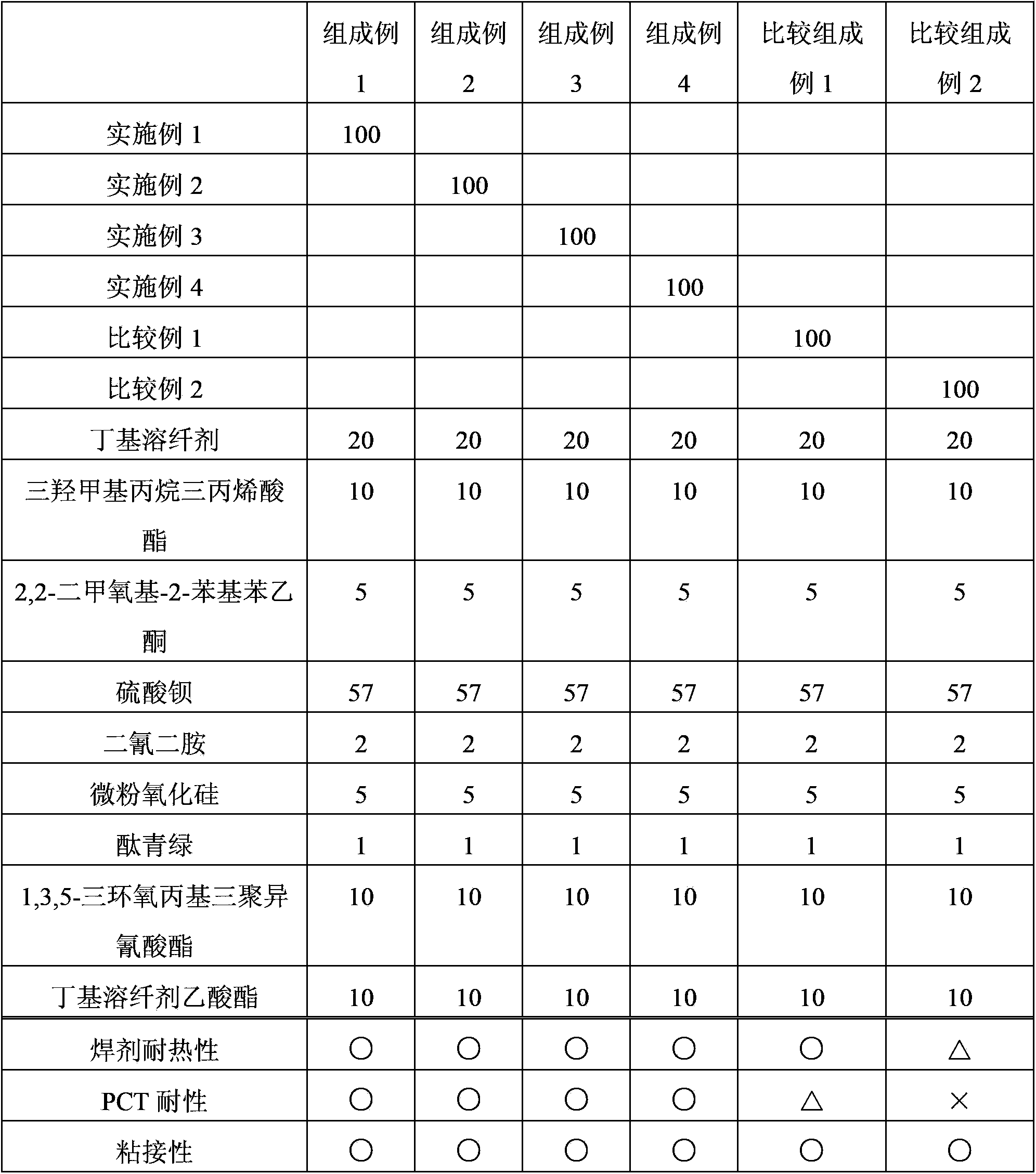
Examples
Embodiment 1
[0047] A novolak-type cresol resin (trade name "Shonol BRG-556") manufactured by Showa High Polymer Co., Ltd. and ring 239 parts of resins having alcoholic hydroxyl groups reacted with ethylene oxide (hydroxyl groups: 239 g / eq., an average of 3.0 moles of alkylene oxide added per 1 equivalent of phenolic hydroxyl groups), 600 parts of methyl methacrylate, Zirconium tetrapentadionate (Zr(acac) 4 ) and 0.2 parts of methylhydroquinone as a polymerization inhibitor, and the reaction was carried out at 100° C. while introducing dry air (50 ml / min). After the start of the reaction, the reaction was carried out for 7 hours while distilling off the generated methanol together with methyl methacrylate from the reaction system. 30 parts of diethylene glycol monoethyl ether acetate were added, and excess methyl methacrylate and methanol were removed by vacuum distillation to obtain a methacrylic group-introduced resin solution. Furthermore, this resin was made to react with 61 parts of...
Embodiment 2
[0055] A methacrylic group-introduced resin solution was obtained in the same manner except that toluene was used instead of diethylene glycol monoethyl ether acetate in Example 1. 6 parts of acetic acid were added to the obtained solution, and it stirred at 40 degreeC for 10 minutes. Next, after washing with water three times, toluene was replaced by 30 parts of diethylene glycol monoethyl ether acetate and distilled off to obtain a methacrylic group-introduced resin solution. Furthermore, this resin was made to react with 61 parts of tetrahydrophthalic anhydrides (0.4 equivalent with respect to alcoholic hydroxyl group), and the curable resin solution which has developability was obtained. The same evaluation as in Example 1 was performed, and the results are shown in Table 1.
Embodiment 3
[0057]In a reaction device made of SUS equipped with a stirrer, a cooling pipe, a thermometer, and an air introduction pipe, a novolak-type cresol resin (trade name "Shonol (シヨウノール) CRG-951" manufactured by Showa High Polymer Co., Ltd.) was placed. ) with propylene oxide reacted with alcoholic hydroxyl resin (hydroxyl group: 386g / eq., per 1 equivalent of phenolic hydroxyl group, an average of 5.0 moles of alkylene oxide added) 386 parts, ethyl acrylate 500 parts, bis Zinc glutarionate (Zn(acac) 2 ) and 0.3 parts of methyl hydroquinone as a polymerization inhibitor were reacted at 100° C. while introducing dry air (50 ml / min). After the reaction started, the resulting ethanol was distilled out of the reaction system together with ethyl acrylate, and reacted for 5 hours. Add 50 parts of diethylene glycol monoethyl ether acetate, carry out vacuum distillation, and remove excess ethyl acrylate and ethanol, thereby obtaining a resin solution through which acrylic acid groups have ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com