High activity paichongding isomers and preparation method thereof
A technology of pifluridin and isomers, which is applied in the field of highly active pifluridin isomers and its preparation, and can solve problems such as drug resistance, lowering the quality of agricultural products, and polluting the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0129] The preparation method of the compound of the present invention
[0130] Compounds of formula A3 and / or formula A4 of the present invention can be prepared by the following methods, but the conditions of the method, such as the amount of reactants, solvents, bases, compounds used, reaction temperature, reaction time, etc. are not limited to the following Explanation. The compound of the present invention can also be conveniently prepared by optionally combining various synthetic methods described in the specification or known in the art. Such a combination can be easily performed by those skilled in the art to which the present invention belongs.
[0131] The preparation method of formula A3 compound and / or formula A4 compound
[0132] The preparation method of the compound of the formula A3 and / or the compound of the formula A4 of the present invention comprises the steps of: splitting the raw material of pichamidin containing the optical isomer shown in the formula A...
Embodiment 1
[0198] Example 1: Chiral HPLC separation to obtain four isomers of pichridam
[0199] Instruments and Conditions
[0200] University liquid chromatograph: Shimadzu, LC-20ATvp, SPD-M20Avp;
[0201] Chromatographic column: CHIRALPAK IC (Daicel, 30×250mm, 5μm);
[0202] Mobile phase: ethanol = 100
[0203] Flow rate: 1-20mL / min;
[0204]Injection volume: 2μL-2mL;
[0205] Detection wavelength: 325nm;
[0206] Column temperature: 20-35°C.
[0207] Experimental procedure
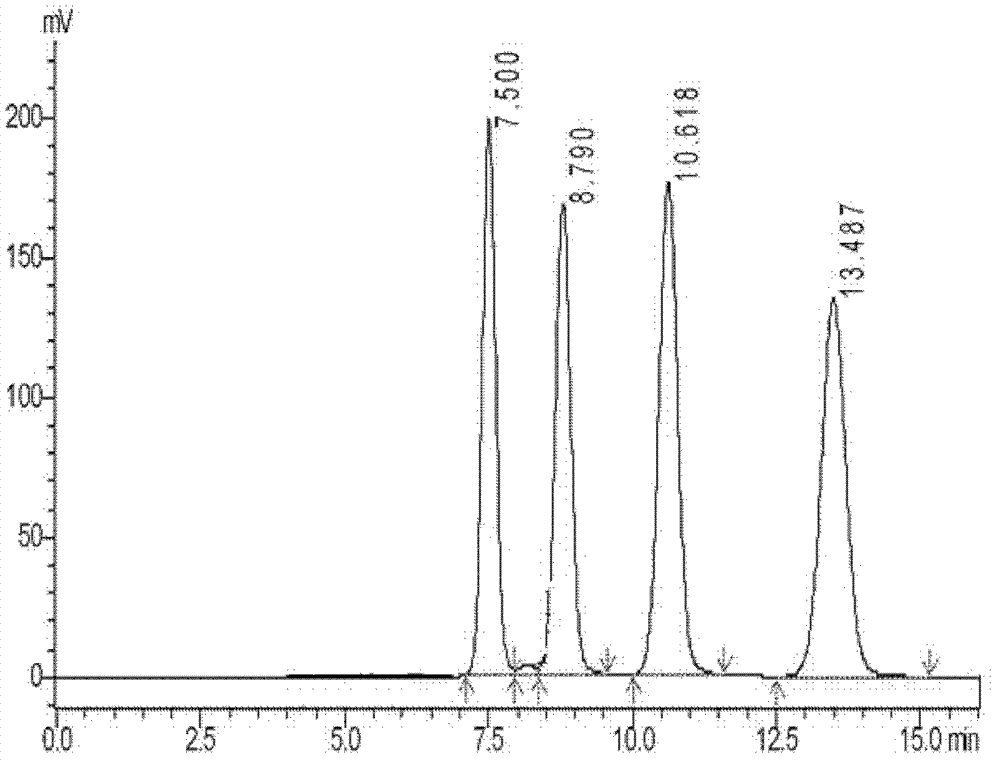
[0208] Prepare sample ethanol solution with pichridam, and prepare the sample solution by liquid chromatography according to the above conditions, collect the target components, take samples for analysis and record the chromatograms. The result is as figure 1 As shown in the figure, from left to right in the figure are the isomers A1, A2, A3 and A4 of piperacrid, and the retention times are 7.500, 8.790, 10.618 and 13.487 min respectively, achieving the baseline separation of the four isomers.
[0209] F...
Embodiment 2
[0214] Example 2: Separation by chiral HPLC to obtain the isomer A34 of piperidin
[0215] Instruments and Conditions
[0216] High performance liquid chromatography: Shimadzu, LC-20ATvp, SPD-M20Avp;
[0217] Chromatographic column: CHIRALPAK IC (Daicel, 30×250mm, 5μm);
[0218] Mobile phase: dichloromethane:methanol:diethylamine=50:50:0.1
[0219] Flow rate: 1-20mL / min;
[0220] Injection volume: 2μL-2mL;
[0221] Detection wavelength: 325nm;
[0222] Column temperature: 20-35°C.
[0223] Experimental procedure
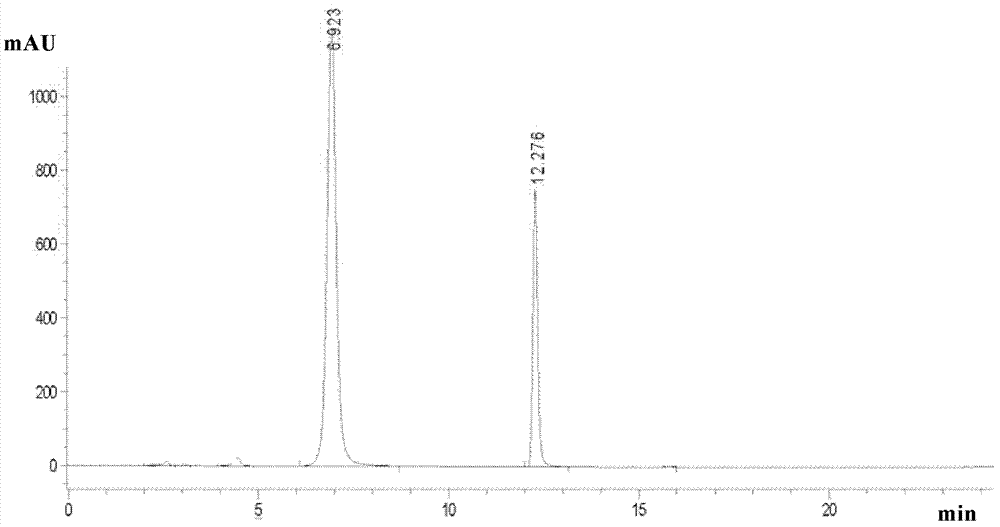
[0224] Take the ethanol solution of the sample configuration of Pichamid, and carry out the liquid chromatography preparation of the university according to the above conditions, collect the target components, take samples and analyze and record the chromatograms, the results are as follows: figure 2 As shown in the figure, from left to right in the figure are the isomers A1, A2, A3 and A4 of pyracrid, the retention times are 3.722, 3.976, 4.666 and 5.624 min...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com