Combination pharmaceutical composition and methods of treating functional diseases or conditions of gastrointestinal tract
A composition and gastrointestinal technology, which can be used in drug combinations, pharmaceutical formulations, nervous system diseases, etc., and can solve problems such as the inability to ensure the efficacy of functional bowel disorder treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
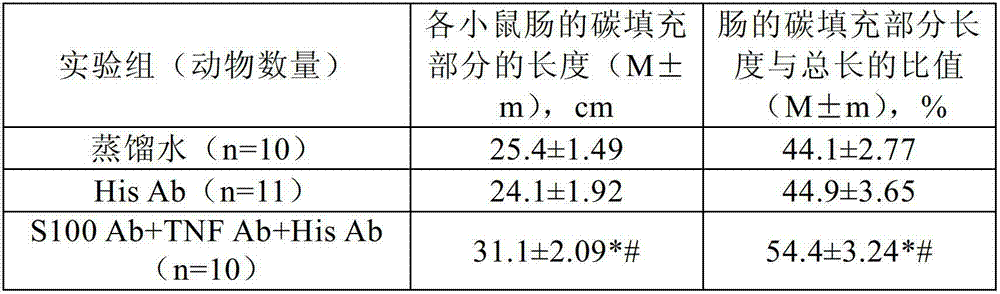
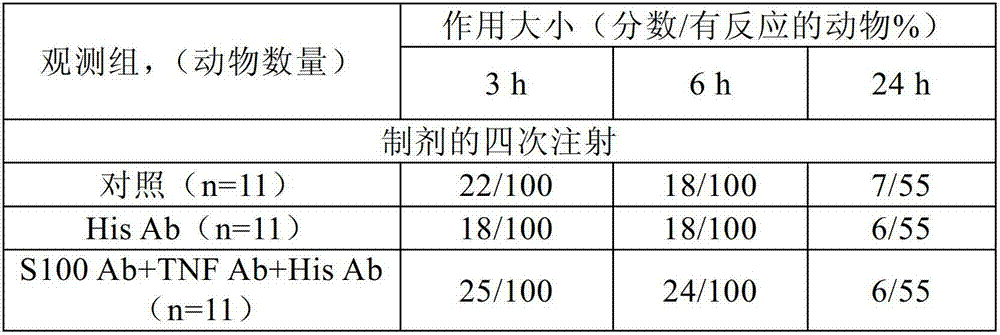
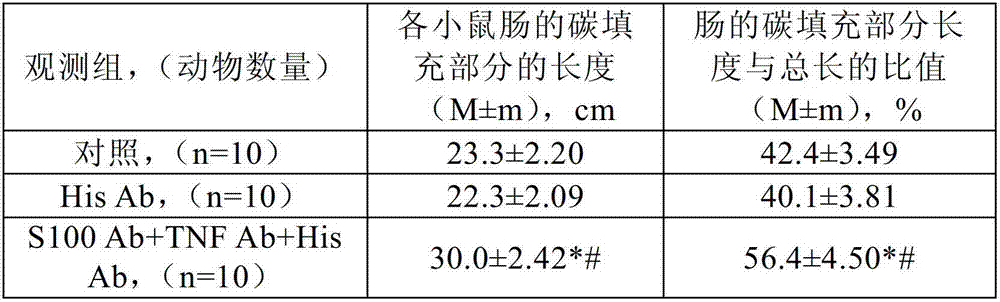
[0277] The effect of the following antibodies was investigated in three experimental studies: i) very low doses of anti-histamine antibody (His Ab), affinity purified with antigen, by high dilution (100 12 、100 30 and 100 200 and ii) a complex (S100Ab+TNF Ab+His Ab) of the following antibodies: a) a very low dose of anti-histamine antibody (His Ab), with antigen Affinity purification was performed by high dilution (100 12 、100 30 and 100 200 diluents (mixture of C12, C30 and C200)), b) a very low dose of anti-S-100 protein antibody (S-100Ab), affinity purified with antigen, by high dilution of the initial matrix solution (100 12 、100 30 and 100 200 Dilutions (mixture of C12, C30 and C200)), and c) very low doses of anti-tumor necrosis factor alpha antibody (TNF Ab), affinity purified with antigen, by high dilution (100 12 、100 30 and 100 200 Diluents (mixture of C12, C30 and C200)) obtained.
[0278] Study 1: Effects on the excretory function of the mouse gastrointes...
Embodiment 2
[0301] Anti-human tumor necrosis factor alpha polyclonal rabbit antibody (TNF Ab), anti-brain-specific protein S-100 polyclonal rabbit antibody (S100Ab) and anti-histamine polyclonal rabbit antibody (His The aqueous-alcoholic solution (6 mg / tablet) of Ab) impregnated the lactose carrier to prepare 300 mg tablets. The components used for impregnation are passed through the initial matrix solution with a concentration of 2.5 mg / ml 100 12 、100 30 、100 200 Obtained by a high dilution of one-fold (a mixture of hundred-fold homeopathic dilutions C12, C30 and C200). For the control group, 300 mg of other tablets were used, saturated with a very low dose of a water-alcoholic solution (3 mg / tablet) of an antihistamine polyclonal rabbit antibody (FisAb) purified with antigen, which Pass the initial matrix solution 100 at a concentration of 2.5 mg / ml 12 、100 30 、100 200 Obtained by a high dilution of one-fold (a mixture of hundred-fold homeopathic dilutions C12, C30 and C200).
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com