Inhalation drug composition with fluticasone propionate and nitric oxide synthase (NOS) inhibitor
A technology of fluticasone propionate and inhaled drug, applied in the field of inhaled pharmaceutical composition, can solve the problems of large irritation, biological cough, inability to form "permissive effect, etc., and achieve the effect of improving airway remodeling and good therapeutic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
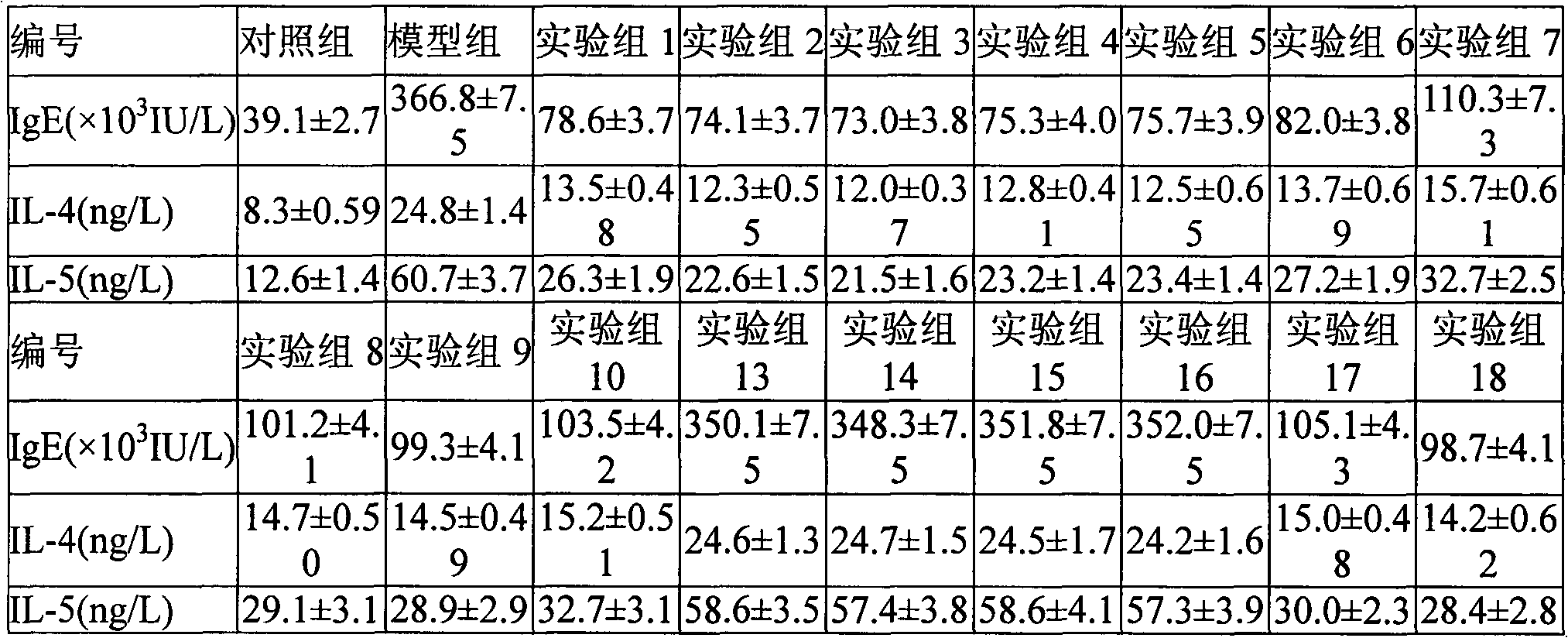
[0040] Fluticasone propionate 80mg, L-N 6 -(1-Iminoethyl)-lysine 600mg dissolved in ethanol, after filtration, the filtrate was spray-dried and micronized to make the average particle size reach 2μm, and 10g of anhydrous lactose was micronized to an average particle size of 20μm by a flow energy mill , after mixing, pass through a 200-mesh sieve for 3 times, mix evenly, and pack in No. 3 capsules. Each capsule contains fluticasone propionate 80 μg, L-N 6 -(1-iminoethyl)-lysine 600 μg.
[0041] The process conditions are: the inlet temperature is 105°C, the outlet temperature is 68°C, the air flow rate is 90%, the inner diameter of the nozzle outlet is 0.1cm, the air flow rate of the nozzle is 800ml / min, and the injection speed is 50mL / h
Embodiment 2-1
[0043] Dissolve 250 mg of fluticasone propionate and 25 mg of N-nitro-L-arginine in ethanol. After filtration, the filtrate is spray-dried and micronized to make the average particle size reach 4 μm. 40 g of anhydrous lactose is micronized to The average particle size is 40 μm, mixed evenly, mixed 3 times with a 200 mesh sieve, and then packed into No. 3 capsules. Each capsule contains fluticasone propionate 250μg and N-nitro-L-arginine 25μg.
[0044] The process conditions are as follows: inlet temperature is 105°C, outlet temperature is 68°C, air flow rate is 90%, nozzle outlet inner diameter is 0.1cm, nozzle air flow rate is 800ml / min, and sample injection speed is 50mL / h.
Embodiment 2-2
[0046] According to the formulation of Example 2-1, the carrier was changed to octaacetate-D-cellobiose octaacetate with an average particle size of 35 μm, and the powder spray was prepared according to the process of Example 2-1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com