Inhalation pharmaceutical composition containing budesonide and terbutaline
A technology for inhaling drugs and terbutaline, which can be used in drug combinations, medical preparations containing active ingredients, drug delivery, etc. Asthma latency, good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
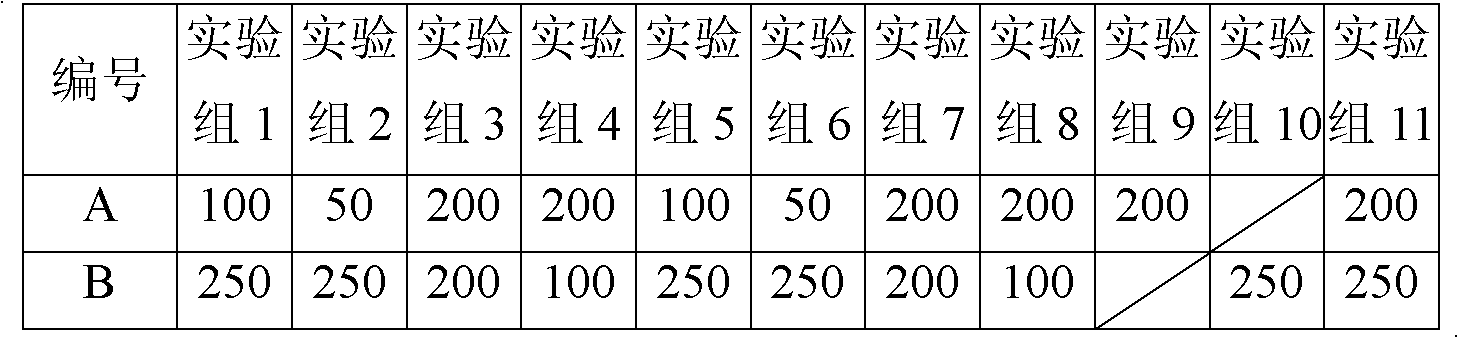
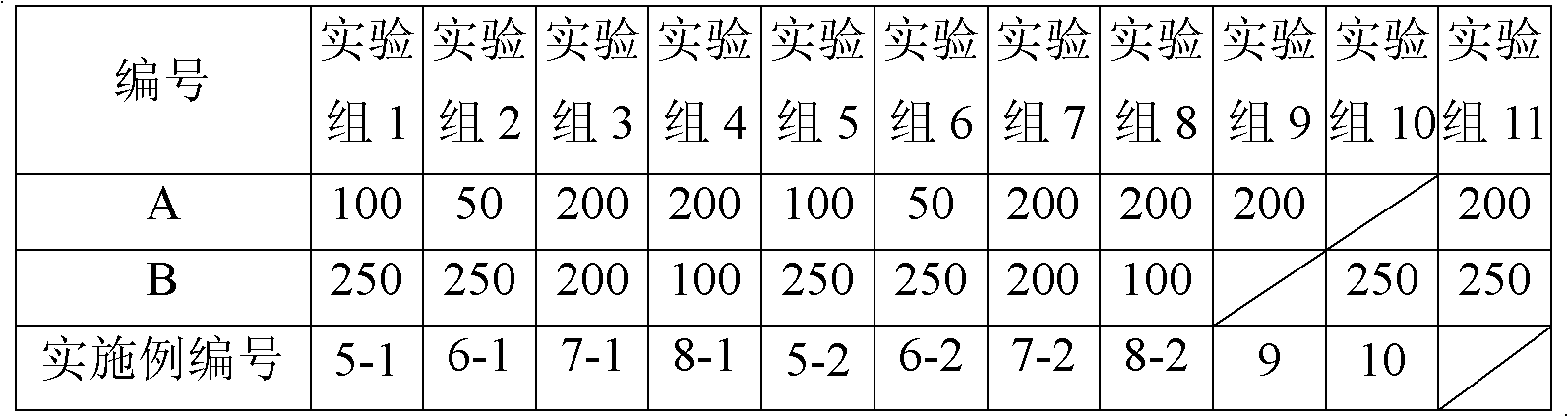
[0041] Example 1-1 (100:250)
[0042] Budesonide 50 mg, dissolved in ethanol, filtered, and the filtrate was spray-dried to make the average particle size reach 5.3 μm (95% particle size range is 4.1-8.0 μm)
[0043] Terbutaline sulfate 250mg was dissolved in ethanol, after filtration, the filtrate was spray-dried to make the average particle size reach 5.6 μm (95% particle size range was 4.5-8.0 μm),
[0044] Budesonide superfine powder 50mg, fully mixed with terbutaline sulfate micropowder.
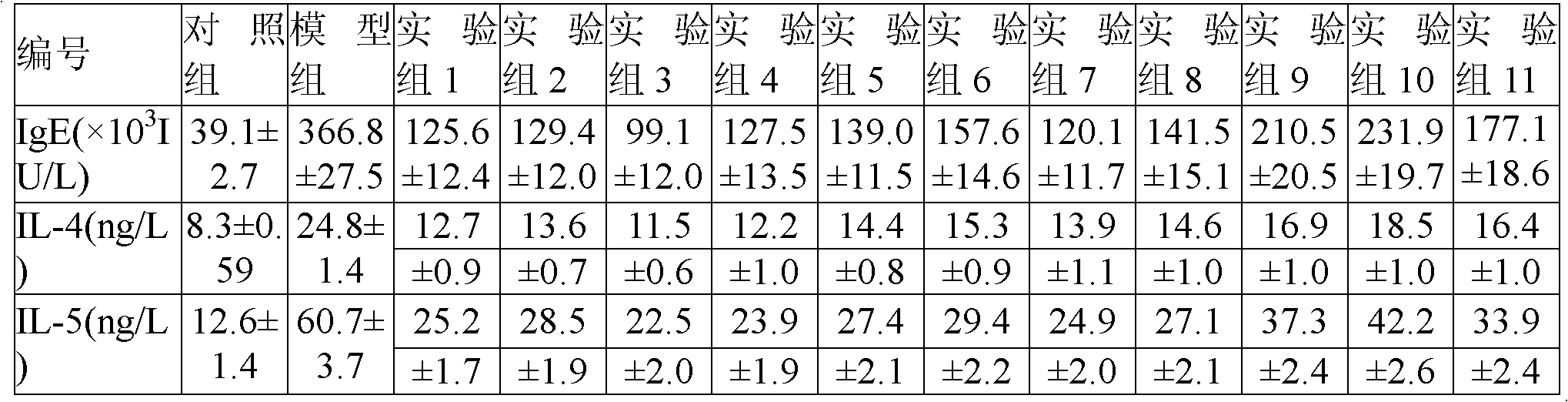
[0045] 20 g of anhydrous lactose was micronized by a flow energy mill to an average particle size of 35 μm, and the mixture of budesonide micropowder, budesonide ultrafine powder and terbutaline sulfate micropowder was added respectively, after mixing, passed through a 200-mesh sieve for 3 times and mixed Divide evenly into No. 3 capsules. Each capsule contains 100 μg of budesonide and 250 μg of terbutaline sulfate.
[0046] The process conditions are as follows: inlet temperature is...
Embodiment 1-2
[0048] According to the formulation and process of Example 1-1, the carrier was changed to octaacetate-D-cellobiose ester with an average particle size of 45 μm. Prepare powder spray.
Embodiment 1-3
[0050] According to the formula and process of Example 1-1, budesonide was changed to micropowder obtained by spray drying. Prepare powder spray.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com