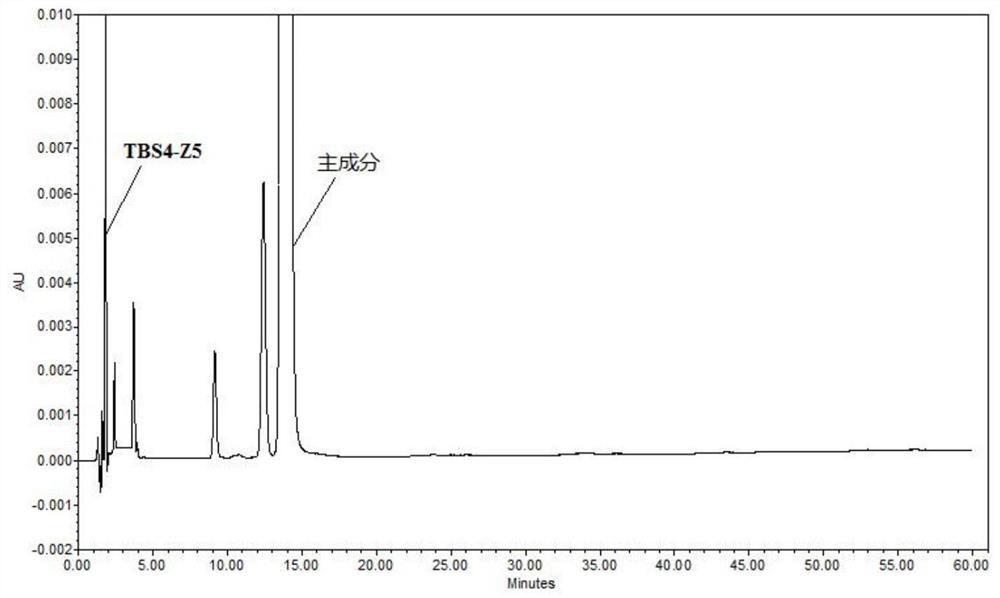
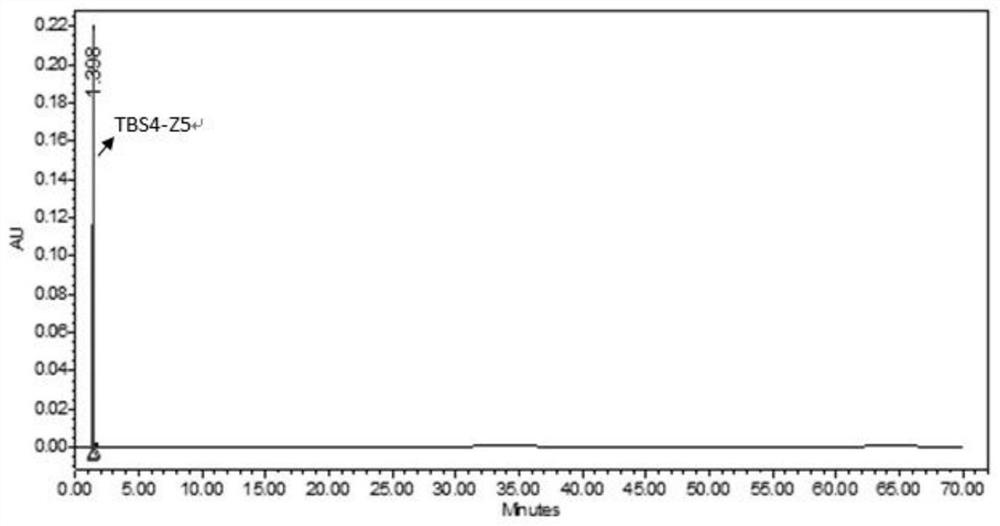
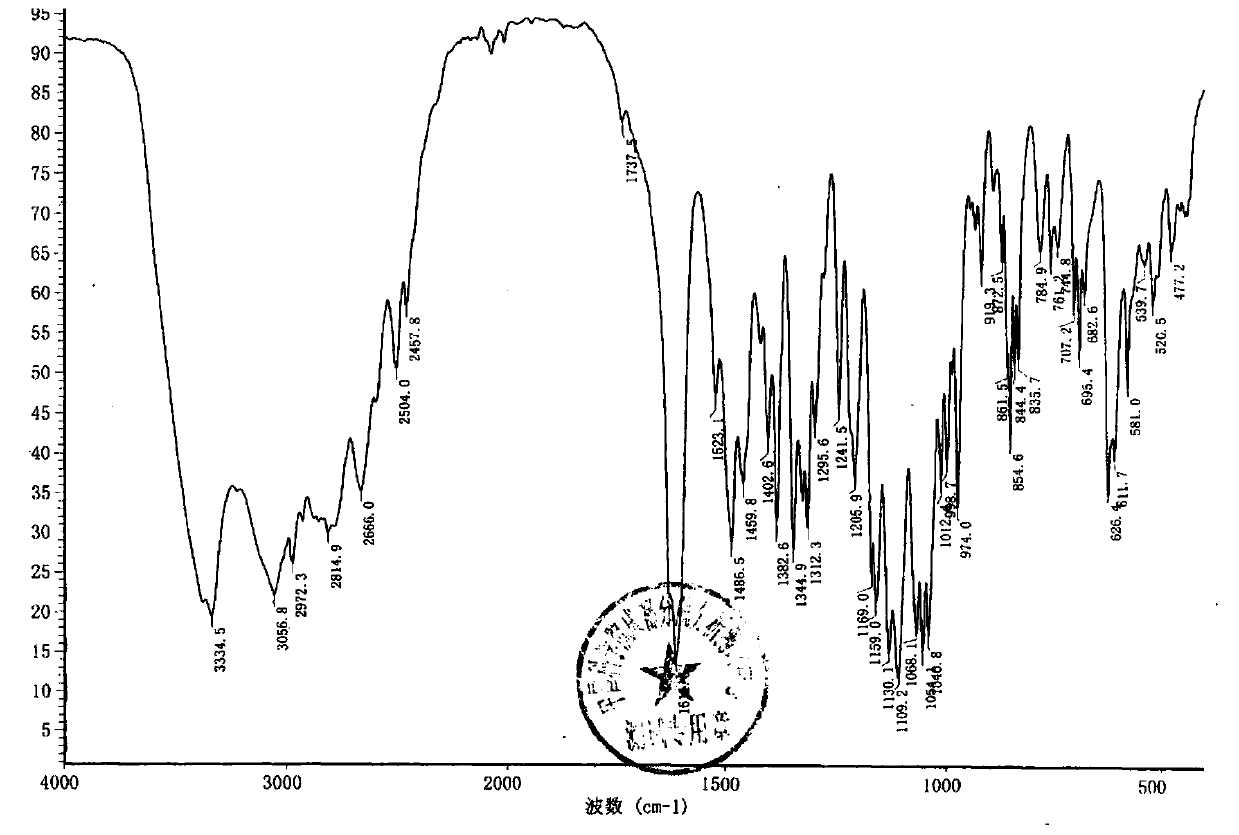
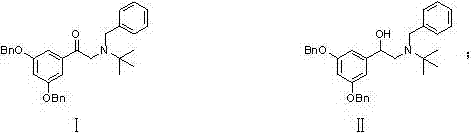Patents
Literature
40 results about "Terbutaline Sulfate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
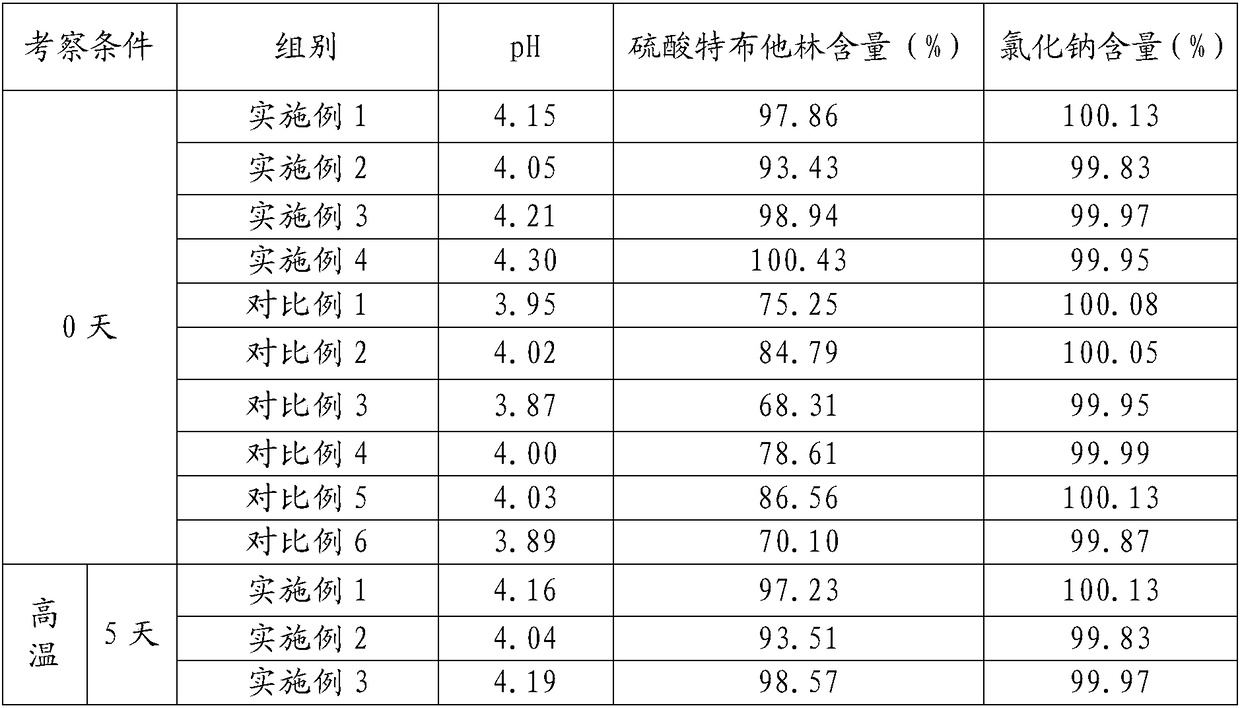
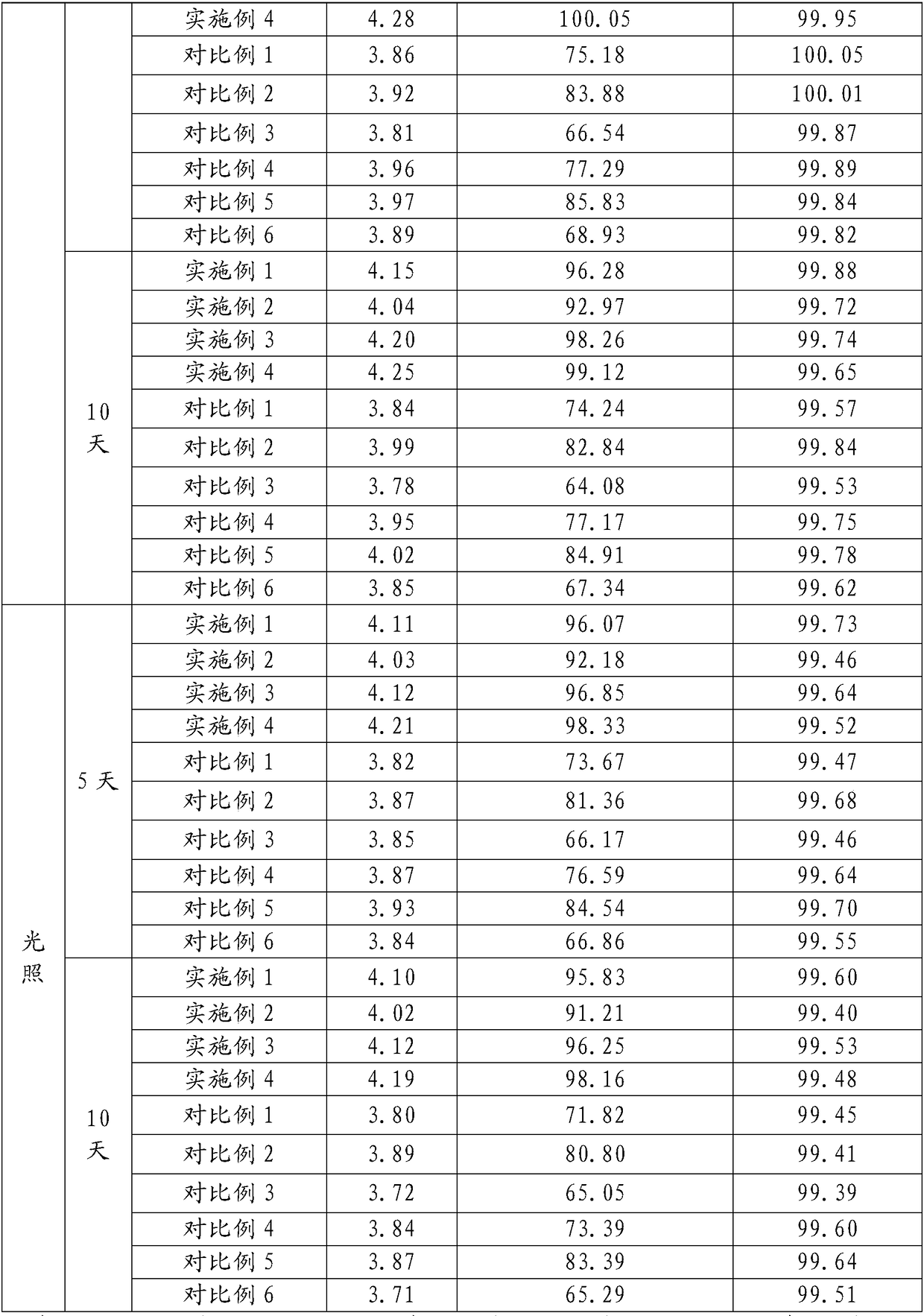
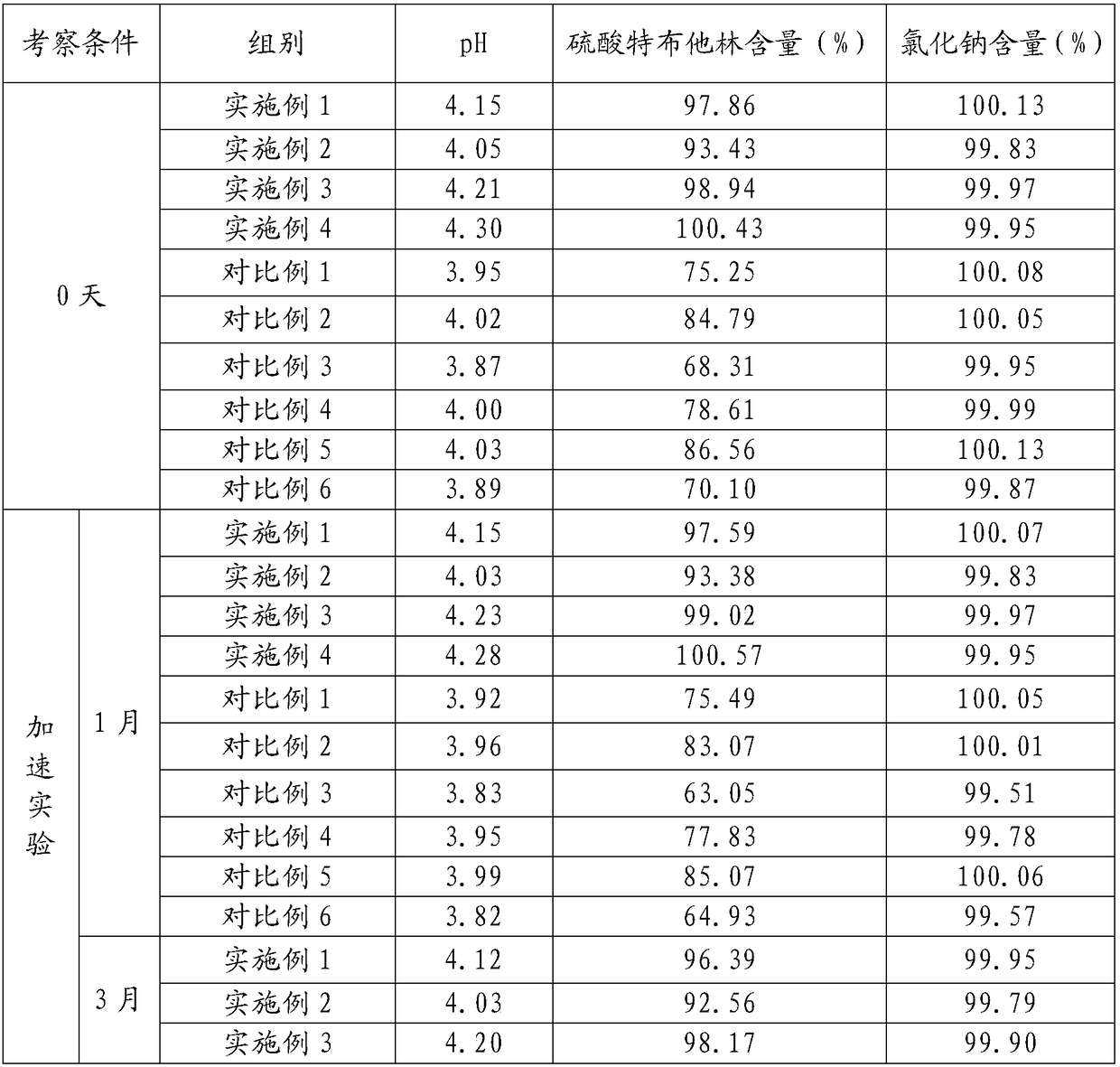
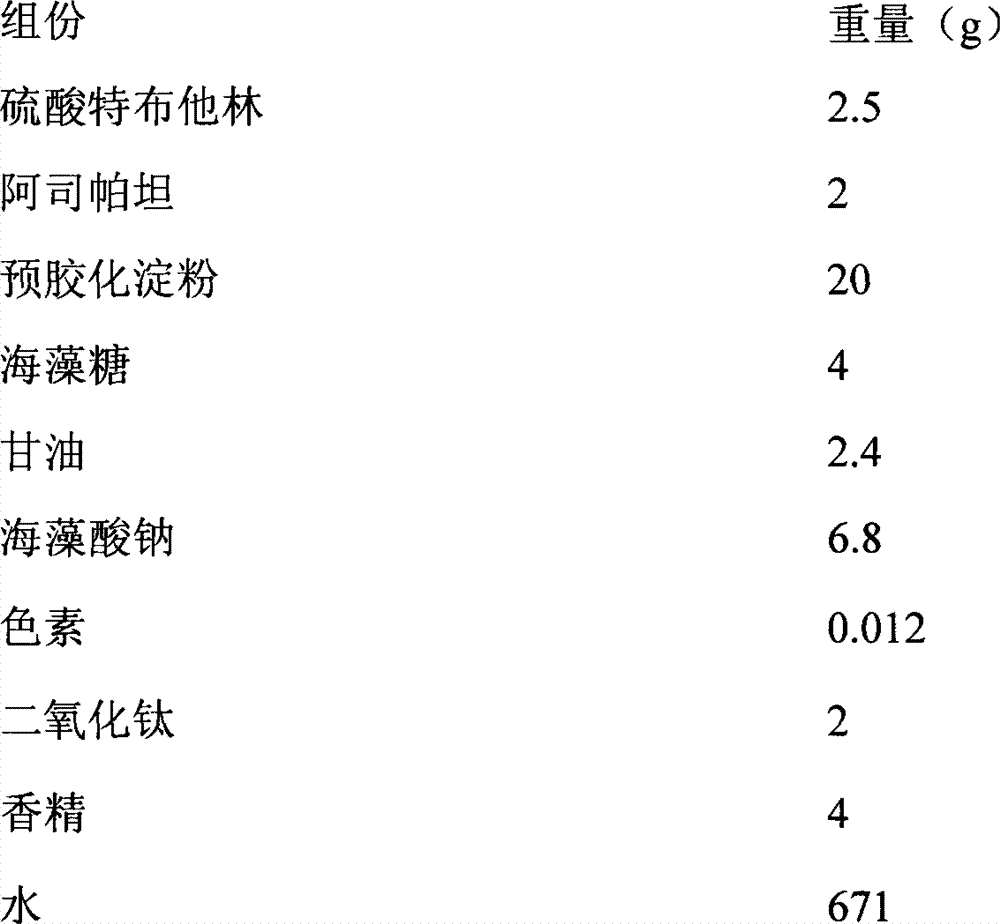
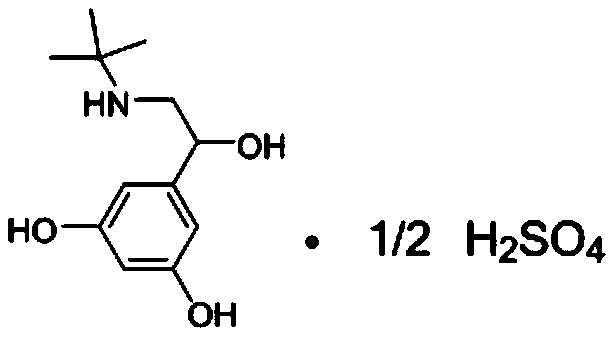
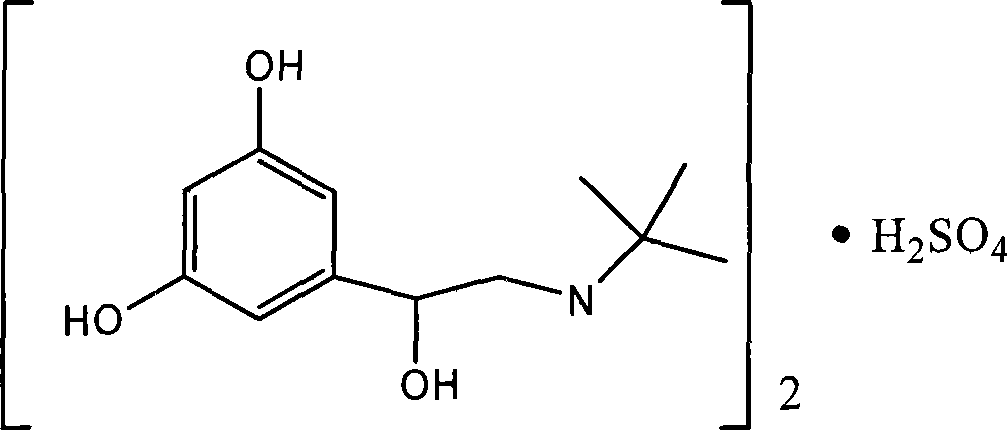
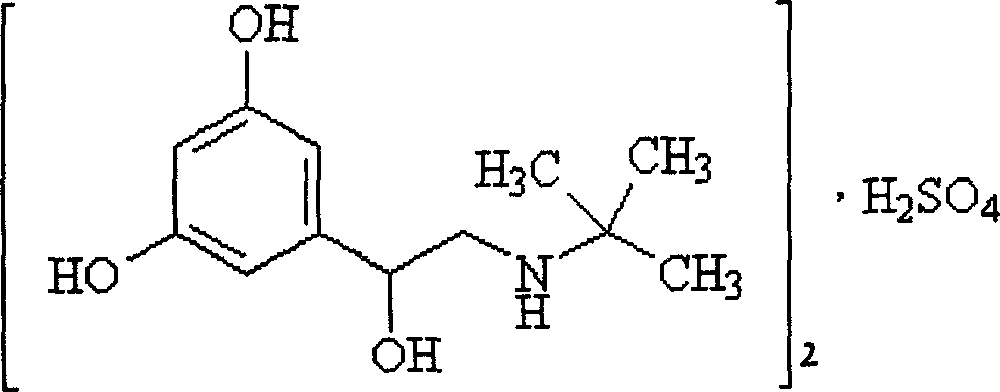
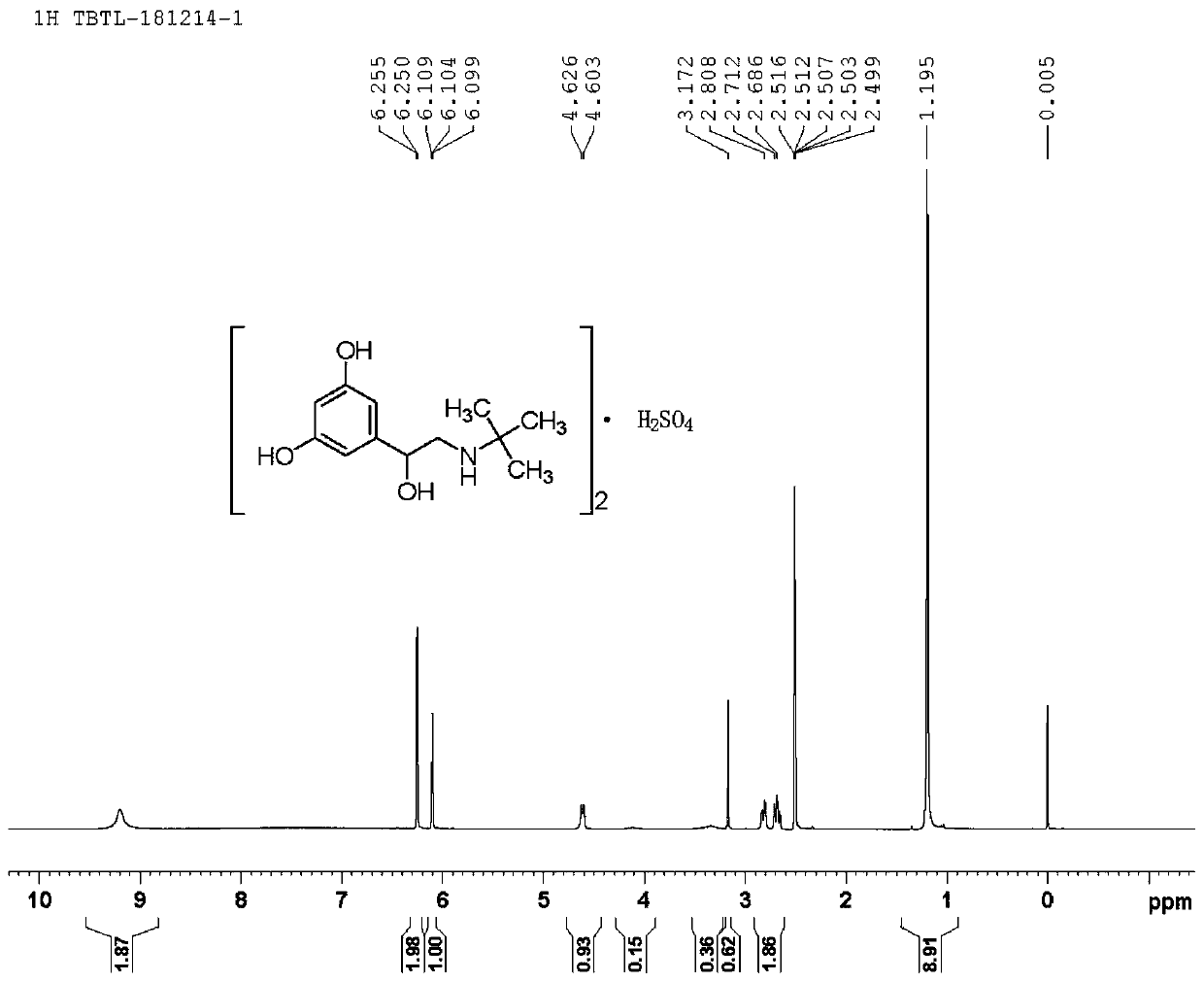
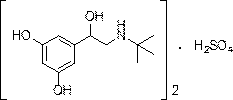
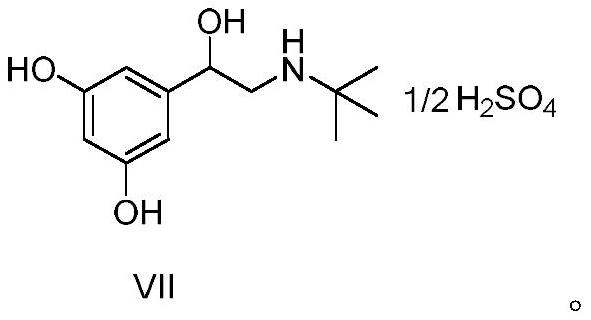
The sulfate salt form of terbutaline, an ethanolamine derivative with bronchodilating and tocolytic properties. Terbutaline sulfate selectively binds to and activates beta-2 adrenergic receptors, leading to intracellular adenyl cyclase activation via a trimeric G protein and subsequent increase in cyclic cAMP production. Increased cAMP levels result in relaxation of bronchial and vascular smooth muscle mediated through the activation of protein kinase A (PKA), which phosphorylates proteins in control of muscle tone. cAMP also inhibits calcium ion release from intracellular stores, reduces calcium entry into cells and induces the sequestration of intracellular calcium all of which aids the relaxation of airway muscles. Terbutaline sulfate also increases mucociliary clearance and reduces release of inflammatory cell mediators.
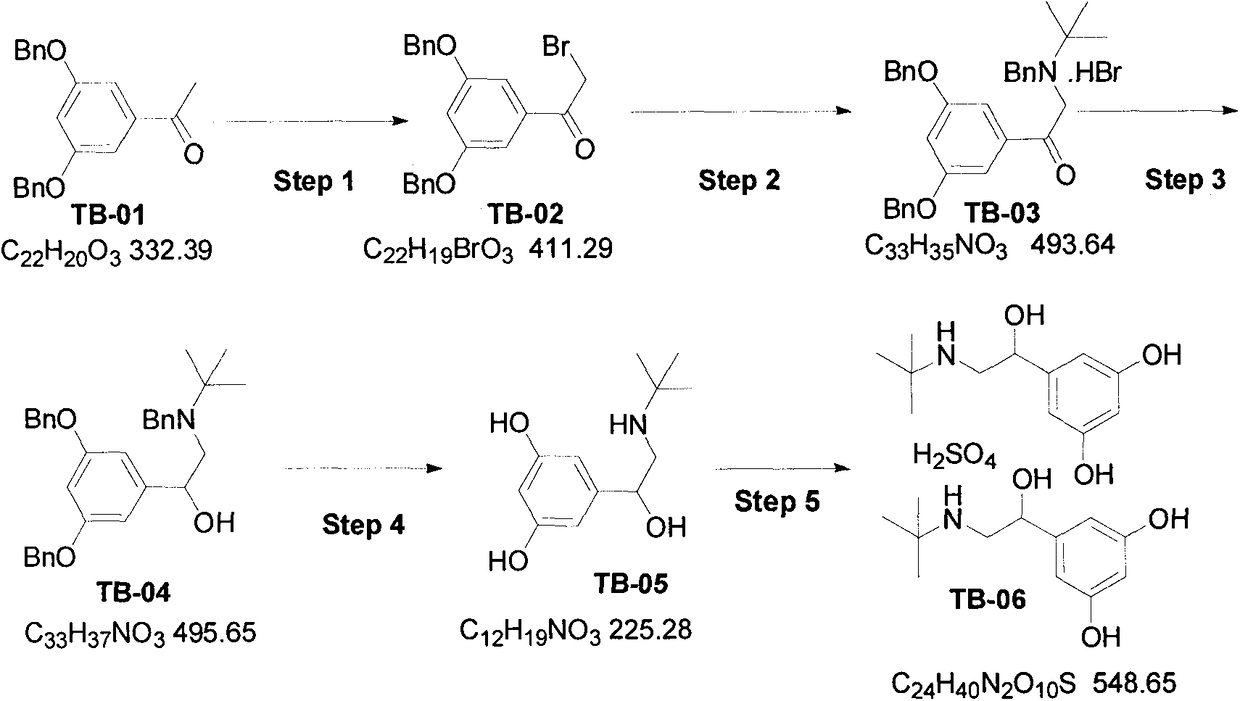
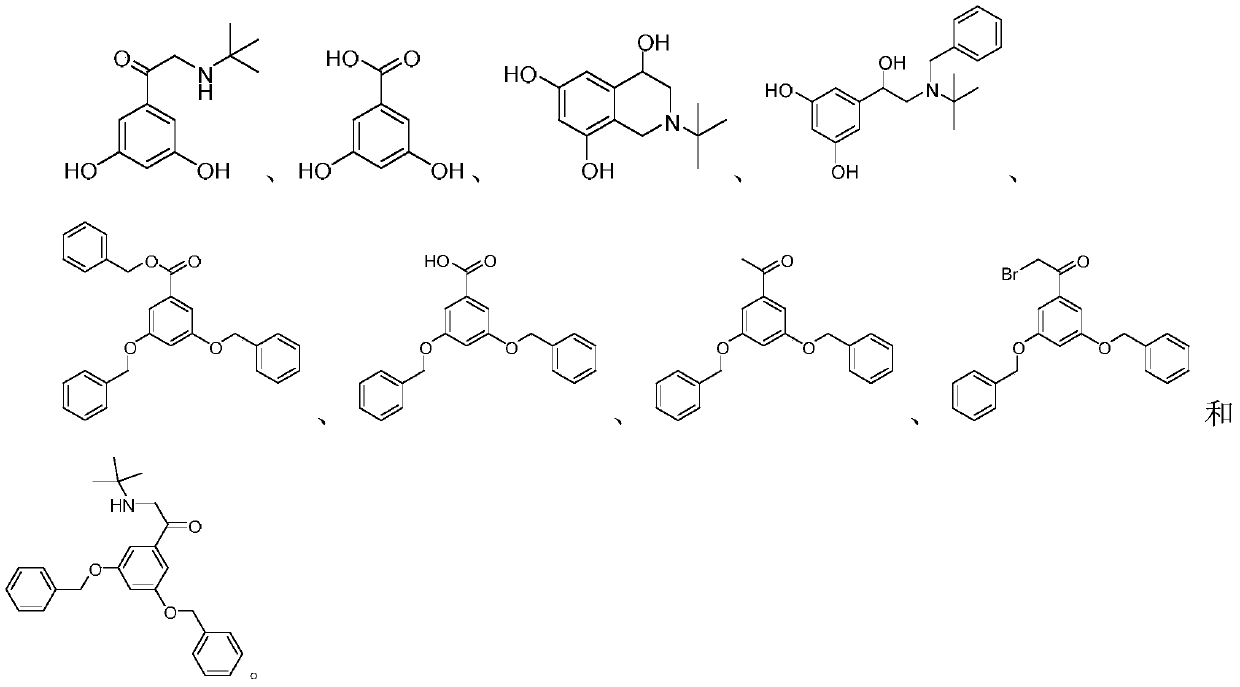
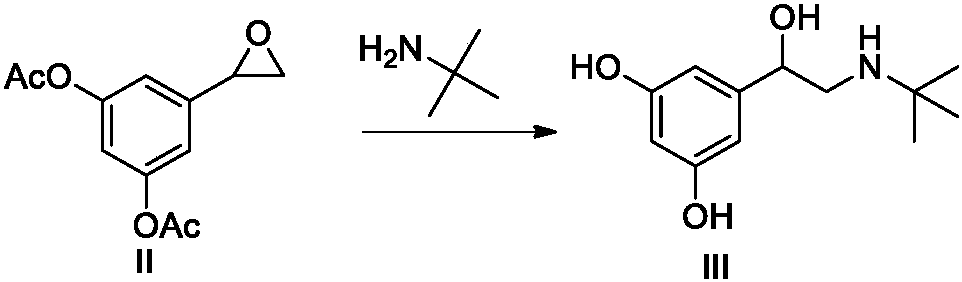
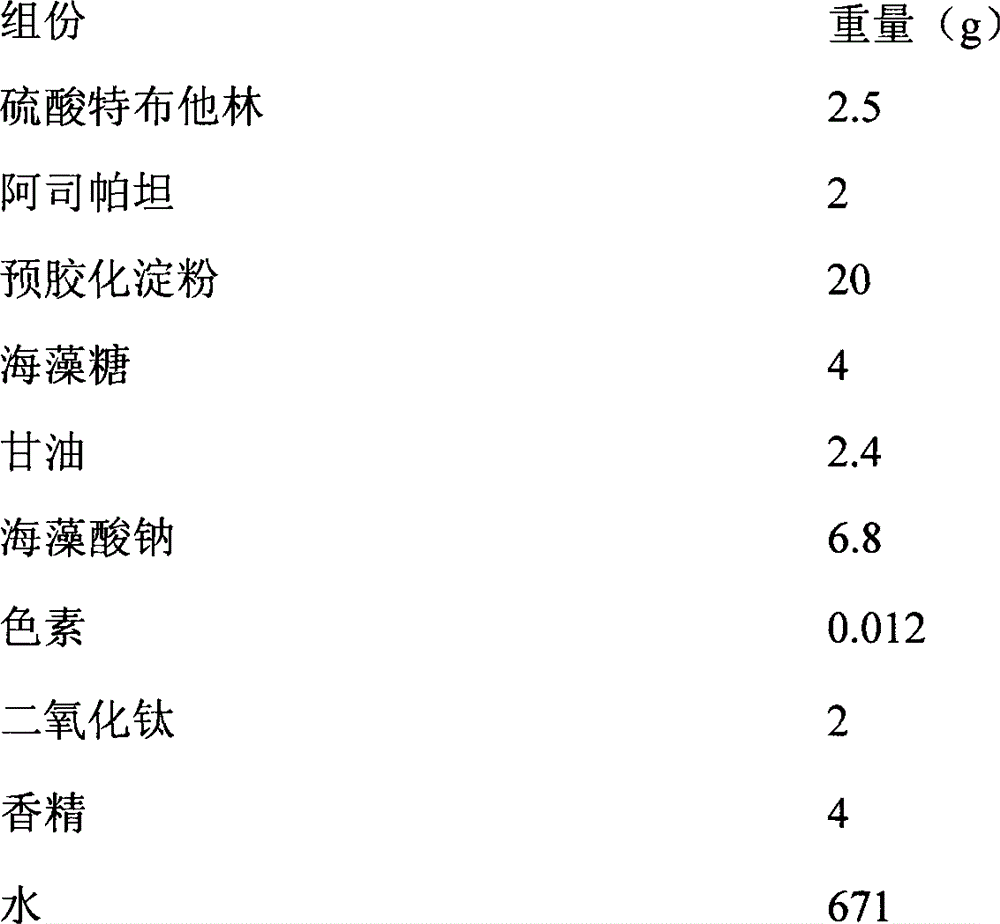
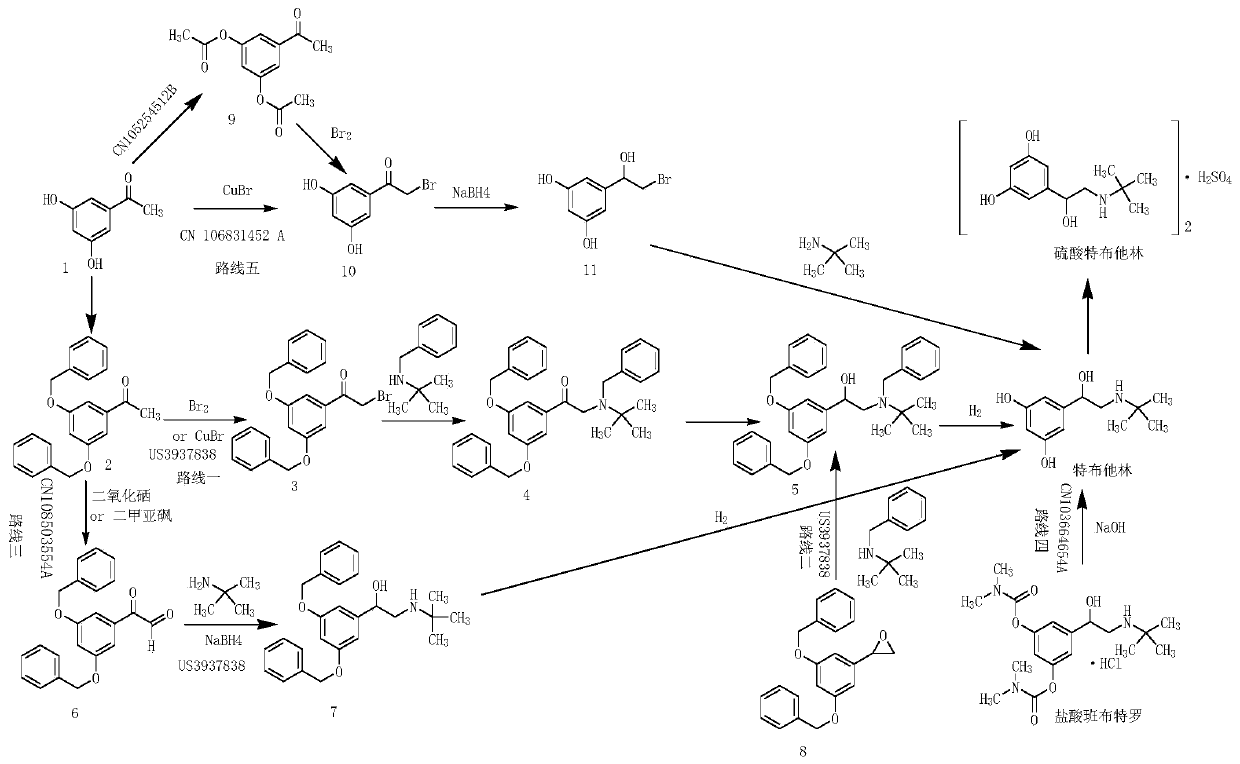
Synthetic method for terbutaline sulfate
InactiveCN108503554AReduce usageReduce pollutionOrganic compound preparationCarbonyl compound preparationTerbutaline SulfateReagent
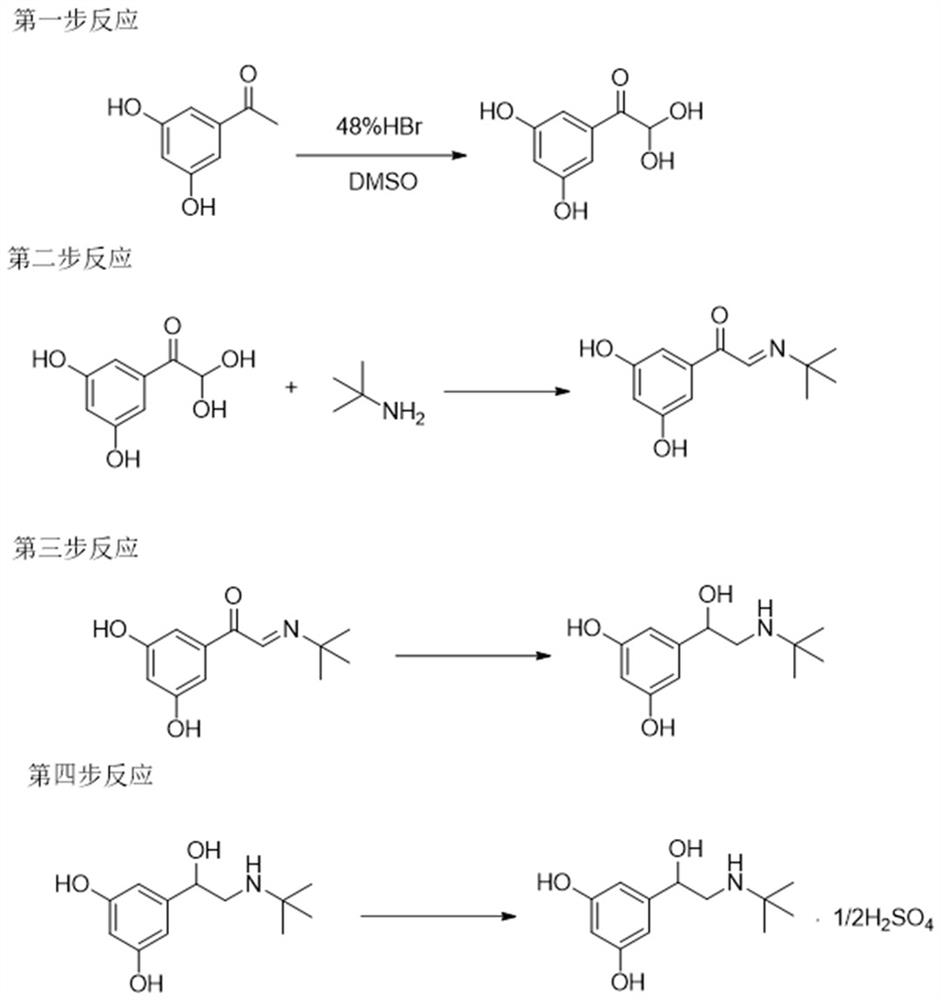
The invention provides a synthetic method for terbutaline sulfate. The method comprises the following specific synthetic route: using 3,5-dihydroxyacetophenone as a raw material, performing benzyl protection, performing oxidization, performing esterification, performing reductive amination, performing debenzylation, and finally performing salt formation with sulfuric acid to obtain the terbutalinesulfate. The preparation method for the terbutaline sulfate provided by the invention has the following beneficial effects: 1, dimethyl sulfoxide (DMSO) is adopted to replace highly-toxic product selenium dioxide, a highly-dangerous reagent is avoided from being used, and the method is safe and high-efficiency; 2, the method does not have a bromination process, and has less pollution to the environment; and 3, the method is easy to operate, has mild reaction conditions, short steps and low costs, and is more suitable for industrialized production.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
Preparation method of terbutaline sulfate
ActiveCN110950765AReduce usageAvoid high temperature and high pressure reactionsOrganic compound preparationCarbonyl compound preparationAcetophenonePhenyl group
The invention discloses a preparation method of terbutaline sulfate. The method comprises the steps: taking 3,5-dihydroxyacetophenone as an initial raw material, firstly, carrying out benzyl protection to obtain 3,4-dibenzyloxyacetophenone, carrying out copper bromide bromination and DMSO oxidation on 3,5-dibenzyloxyacetophenone to obtain 3,5-dibenzyloxyacetophenone aldehyde, carrying out reductive amination on 3,5-dibenzyloxyacetophenone aldehyde and tert-butylamine to generate 1-[3,5-di(benzyloxy)phenyl]-2-(tert-butylamino)ethanol, and finally carrying out hydrogenation debenzylation and sulfuric acid salification, to obtain terbutaline sulfate. Compared with the prior art, the method has the advantages that the used raw materials and auxiliary materials are cheap and easily available, the use of high-risk and highly-toxic reagents is avoided, high-temperature and high-pressure reaction is avoided, the operation is simple and convenient, the reaction conditions are mild, and the defects of long steps, low yield, potential safety hazards and the like in the prior art are overcome.
Owner:ZHEJIANG PHARMA COLLEGE
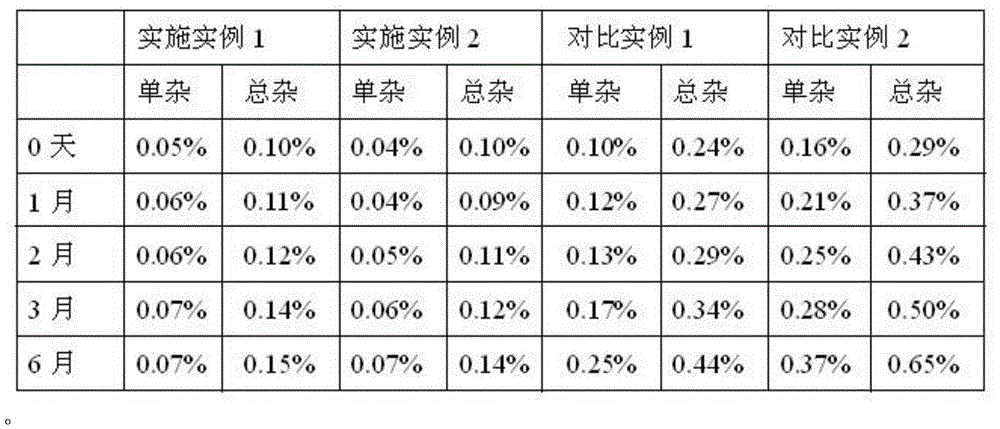
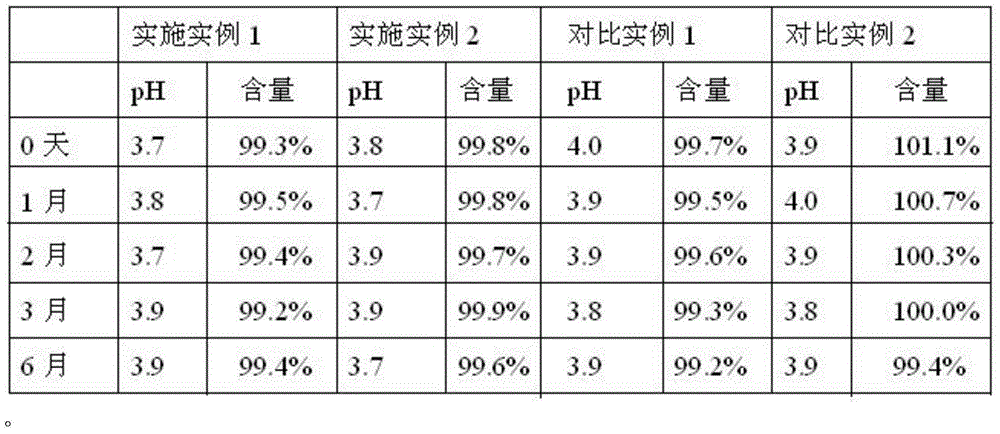
Processing method for improving stability of terbutaline sulfate injection and terbutaline sulfate injection
ActiveCN108078918AImprove stabilityStable contentOrganic active ingredientsInorganic non-active ingredientsNitrogenSilanization
The invention relates to a processing method for improving the stability of a terbutaline sulfate injection. The processing method comprises the following steps: S1, passivating a stainless steel preparation tank and a pipeline by using nitric acid of 8 wt% before preparing the terbutaline sulfate injection, wherein the passivating temperature is 49 DEG C to 52 DEG C, the passivating time is 0.5-2hours; washing with water for injection until the pH is 5.0-7.0; S2, putting the pipeline passivated by the terbutaline sulfate injection prepared through the passivated stainless steel preparation tank in a glass bottle under the protection of nitrogen, and sterilizing, wherein the inner surface of the glass bottle is modified by an active group, and the active group is one or more of amino, sulfydryl, carboxyl and anhydride. The stability of the terbutaline sulfate injection is greatly improved through passivating and silanization, so that the content of the terbutaline sulfate is kept stable before and after filling and in the long-term storage process.
Owner:石药银湖制药有限公司
Terbutaline sulfate oral instant film and preparation method thereof
InactiveCN102961365APleasant tasteImprove palatabilityOrganic active ingredientsPharmaceutical non-active ingredientsSevere disabilityBULK ACTIVE INGREDIENT
The invention discloses a terbutaline sulfate oral instant film and a preparation method thereof, and belongs to the technical field of medicines. The terbutaline sulfate oral instant film comprises active ingredients, such as terbutaline sulfate, film-forming materials, disintegrating agents and opacifying agent with effective dosages, and necessary pharmaceutically acceptable filling agents, sweetening agents and wetting agents; and coloring agents are added according to requirements. The terbutaline sulfate oral instant film is good in palatability, fast in response, small in size, and suitable for children, elders, completely-bedridden persons and patients with severe disability; the film can be rapidly dissolved in a disintegrating mode in the oral cavity; water is not needed to orally take; and people can take the medicines any time under a condition that water is inconvenient to drink.
Owner:天津市聚星康华医药科技有限公司
Preparation method of high-purity injection-grade terbutaline sulfate
InactiveCN109305920AAddressing risks with a high risk of contaminationLow costOrganic compound preparationCarbonyl compound preparationSubstitution reactionCarbonyl reduction
The invention provides a preparation method of high-purity injection-grade terbutaline sulfate. The preparation method is characterized in that 3,5-dibenzyloxyacetophenone is adopted as a raw material, a bromination reaction, a substitution reaction, carbonyl reduction and hydrogenation reduction are conducted, salifying with sulfuric acid is conducted, and the terbutaline sulfate is obtained. A synthetic route is shown in the description.
Owner:HAINAN LEVTEC PHARMA
Terbutaline sulfate injection and preparation method thereof
PendingCN112826793AAvoid influenceImprove stabilityOrganic active ingredientsInorganic non-active ingredientsInjection solutionAmpoule
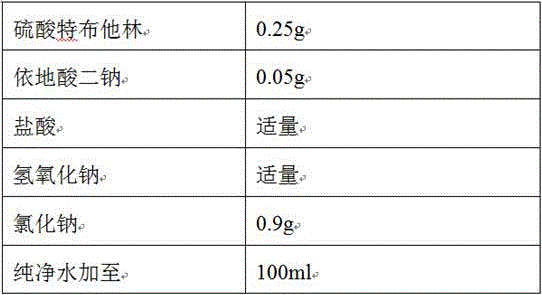
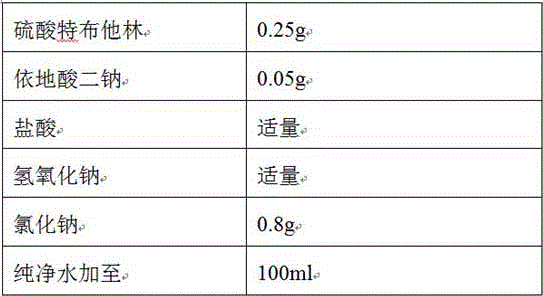
The invention relates to the technical field of pharmaceutical preparations, and particularly discloses a terbutaline sulfate injection and a preparation method thereof. The preparation tank is subjected to complexing treatment by using a sodium ethylene diamine tetracetate solution, and after complexing is completed, the preparation tank is flushed by using water for injection until the pH value is 5.0-7.0 and the electrical resistivity is less than 0.5, so that the preparation tank can be used for preparing the injection; and in the preparation process, the water for injection is filled with nitrogen, the terbutaline sulfate is added after dissolved oxygen in the water is controlled to be lower than 100 ppb, and the headspace residual oxygen content in an ampoule bottle is controlled to be smaller than 2% in cooperation with nitrogen introduction in the filling process, so that the influence of oxygen and metal ions on the terbutaline sulfate is effectively avoided. The terbutaline sulfate injection provided by the invention is simple in prescription, and the stability of terbutaline sulfate is remarkably improved on the premise of not introducing other auxiliary materials such as an antioxidant or a complexing agent, a stabilizer and the like, so that the clinical application safety is improved.
Owner:SHIJIAZHUANG NO 4 PHARMA
Stable terbutaline sulfate injection and preparation process thereof
InactiveCN104523582AOrganic active ingredientsInorganic non-active ingredientsNitrogenIntravenous fluid
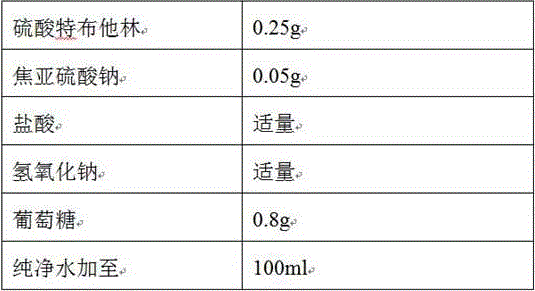
The invention discloses a stable terbutaline sulfate injection, wherein 1 ml of the stable terbutaline sulfate injection comprises 0.1-0.4mg of terbutaline sulfate, 8.0-10mg of sodium chloride and the balance of water for injection. In the preparation process of the stable terbutaline sulfate injection, nitrogen is used for protection so that the using of antioxidant can be decreased; low-temperature preparation is adopted to control the stability of the product so that the impurity stability after steam sterilization is ensured and the metal ion complexing agent can be decreased. According to the stable terbutaline sulfate injection and the preparation process thereof, the problem of adding other auxiliary materials to maintain the stability of the preparation in a clinic intravenous fluid is solved and the clinic safe medication is ensured.
Owner:CHENGDU SINO STRONG PHARMA
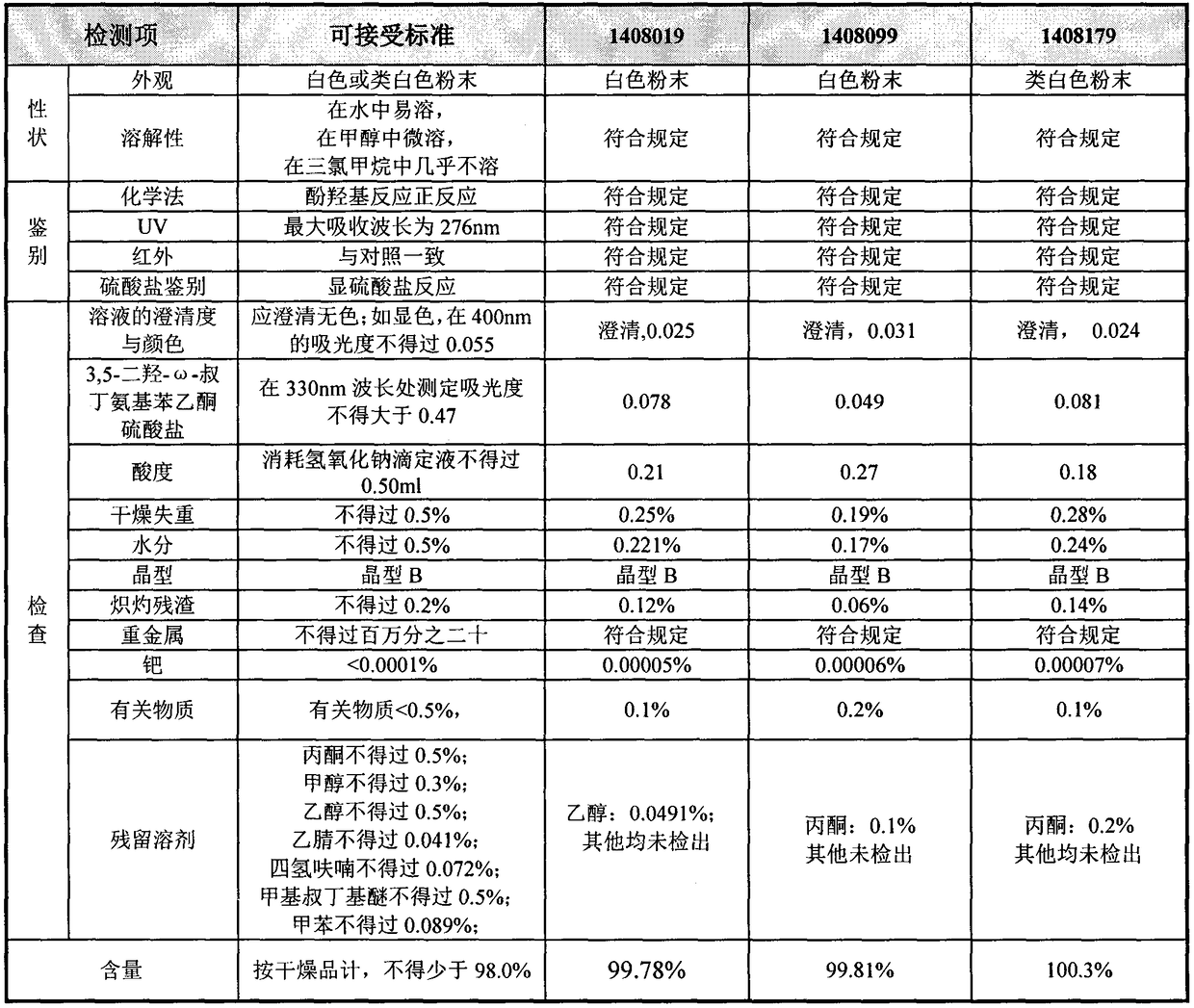
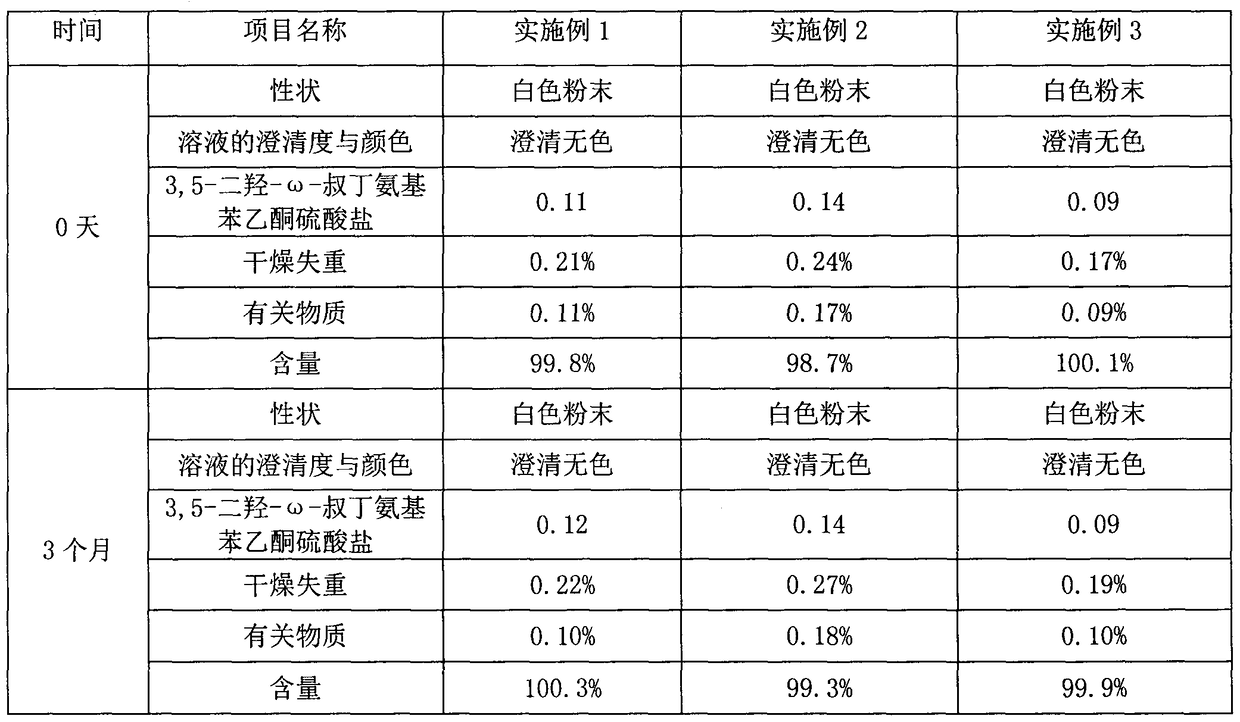
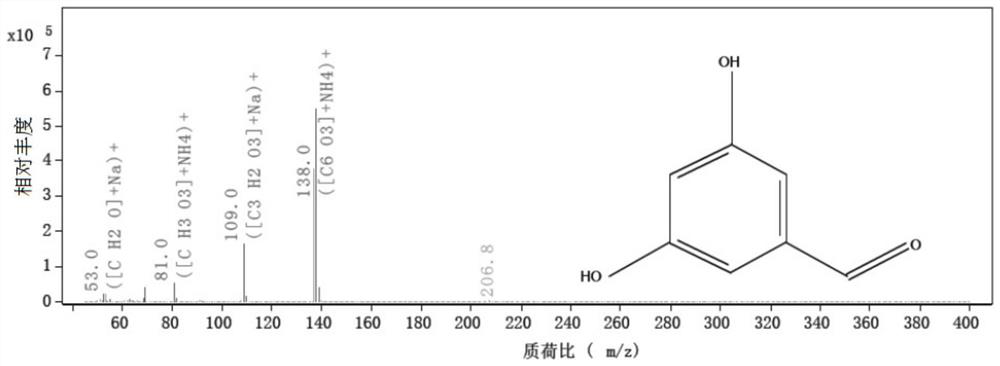
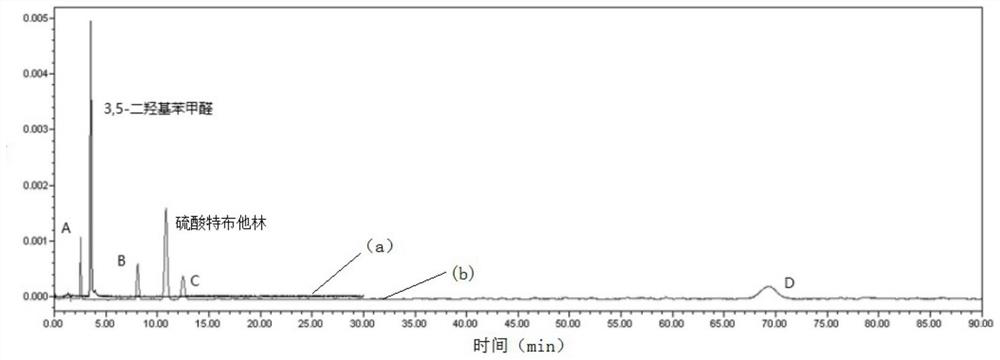
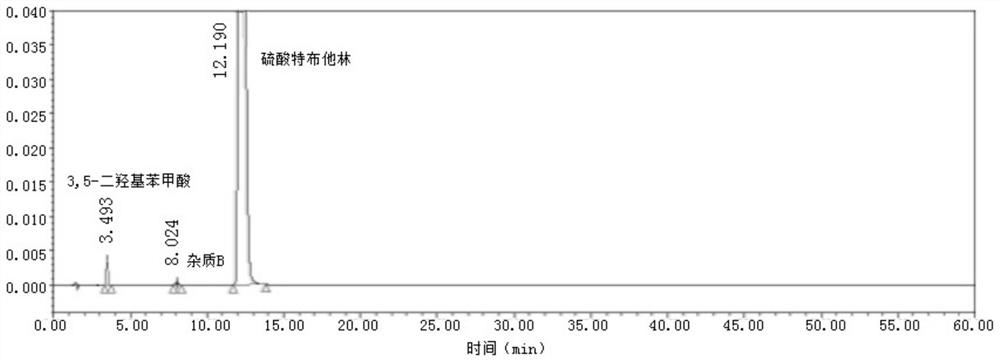
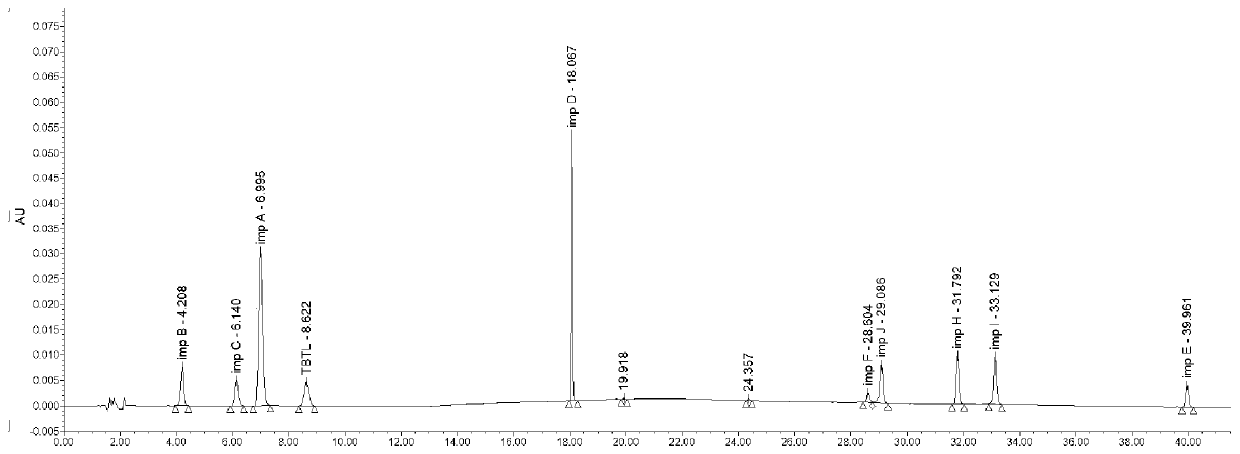
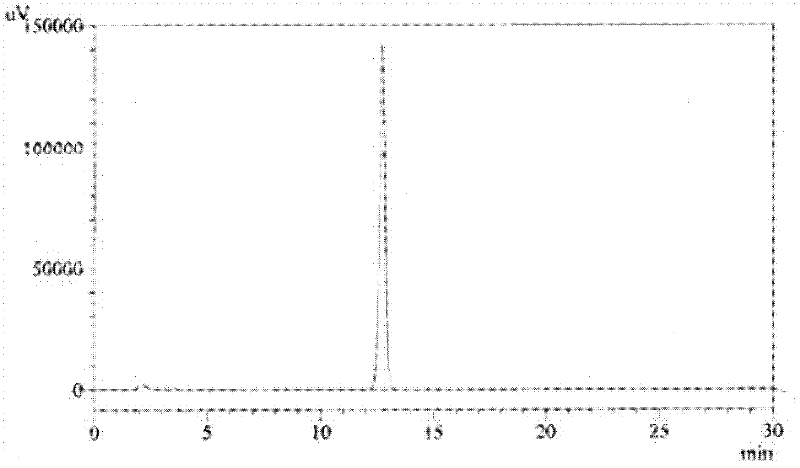
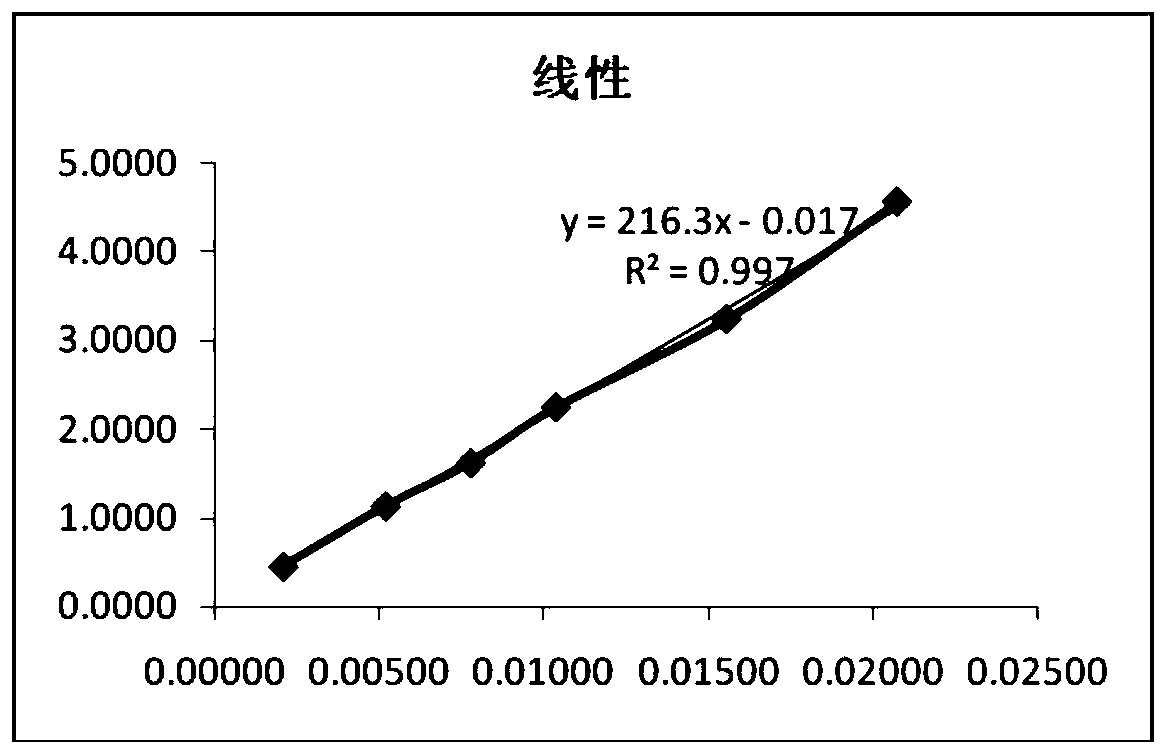
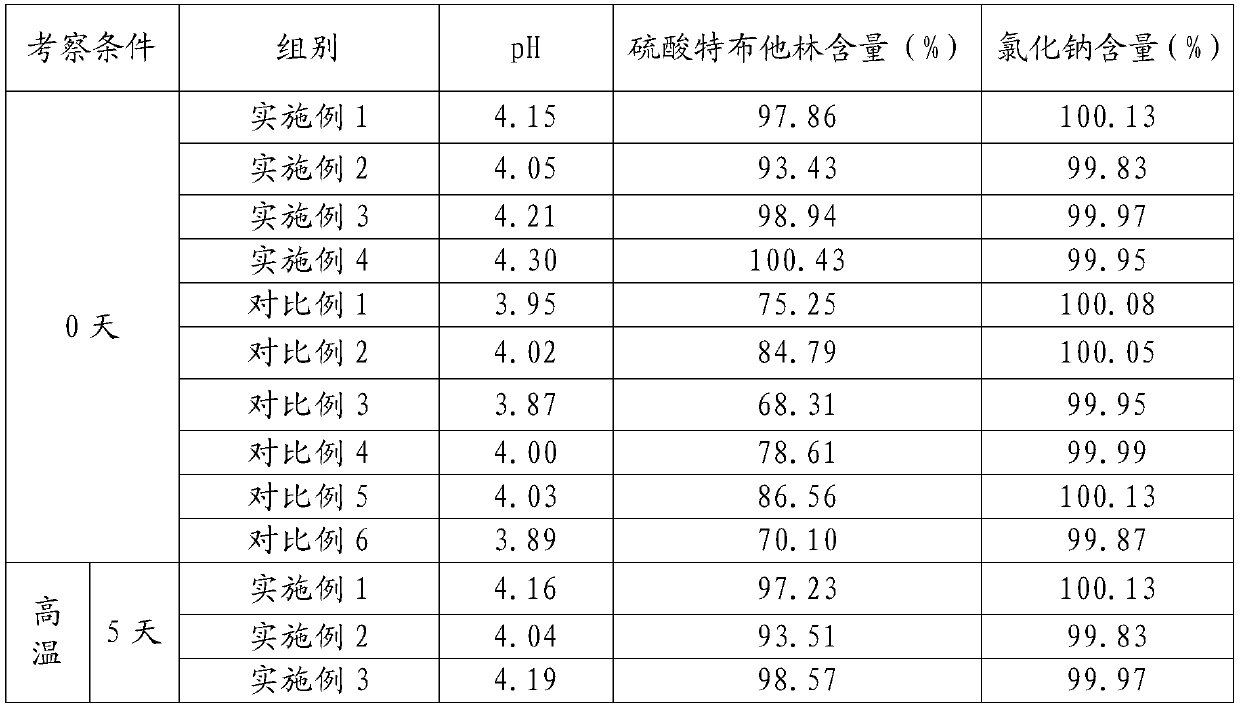
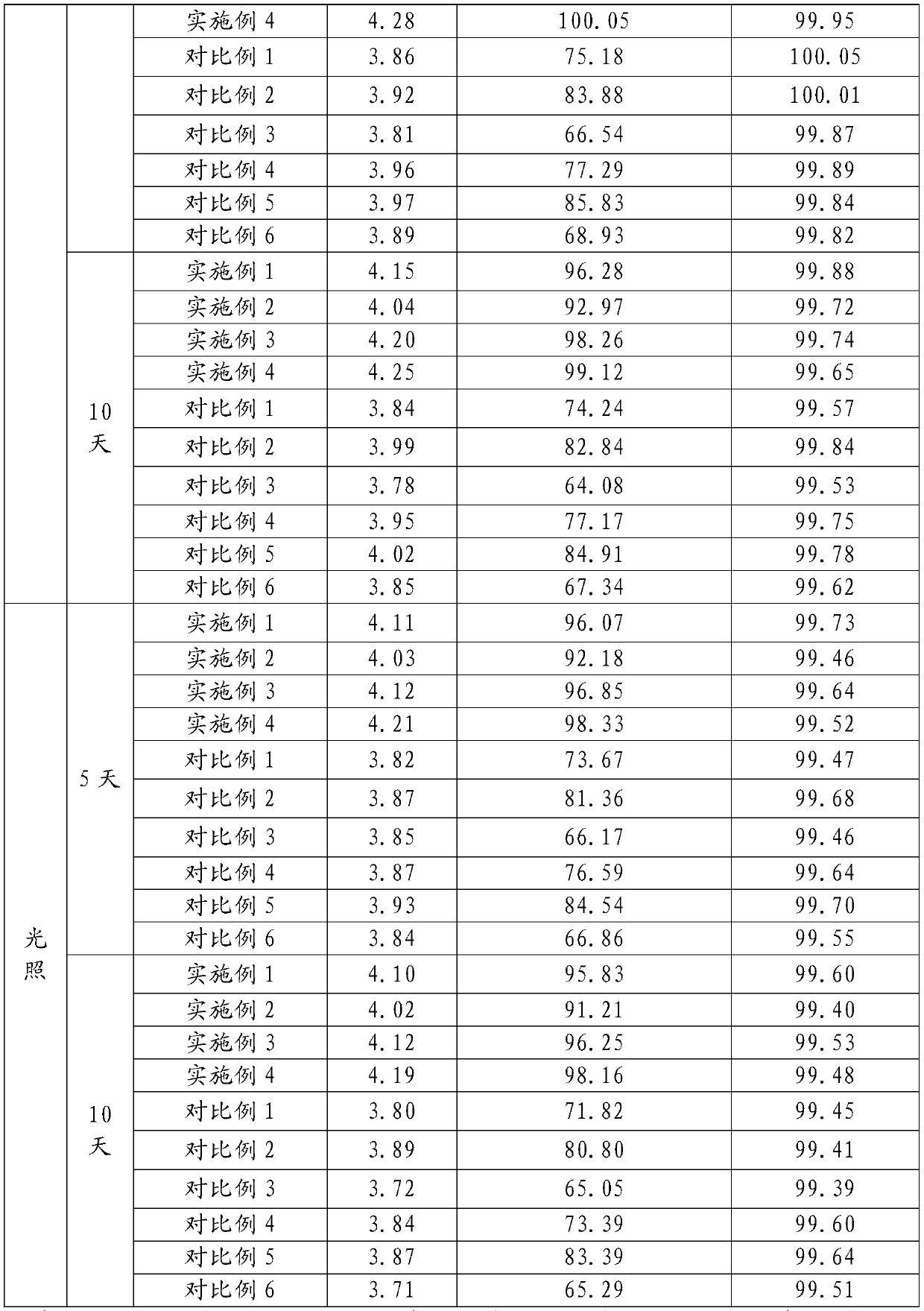
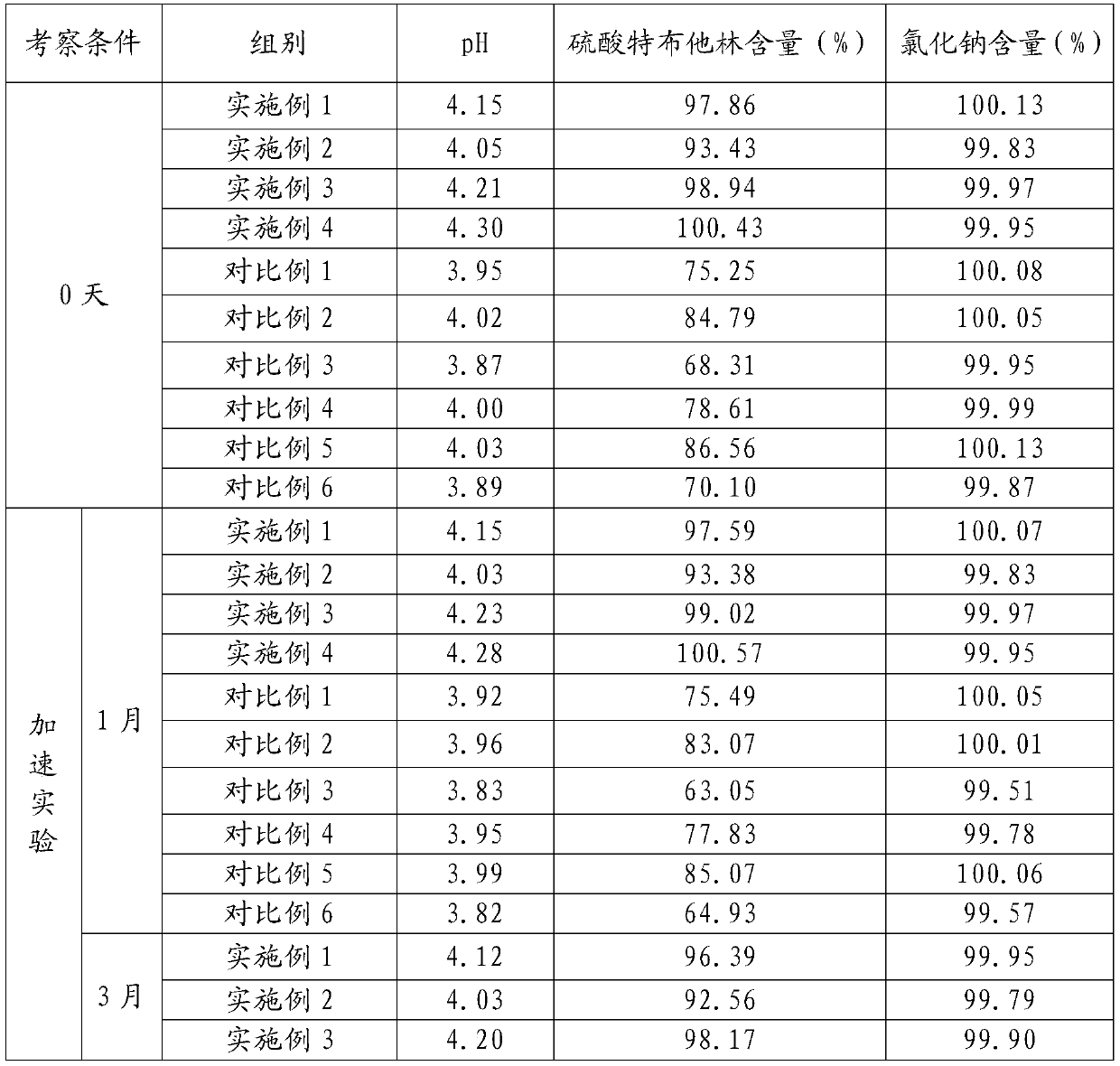
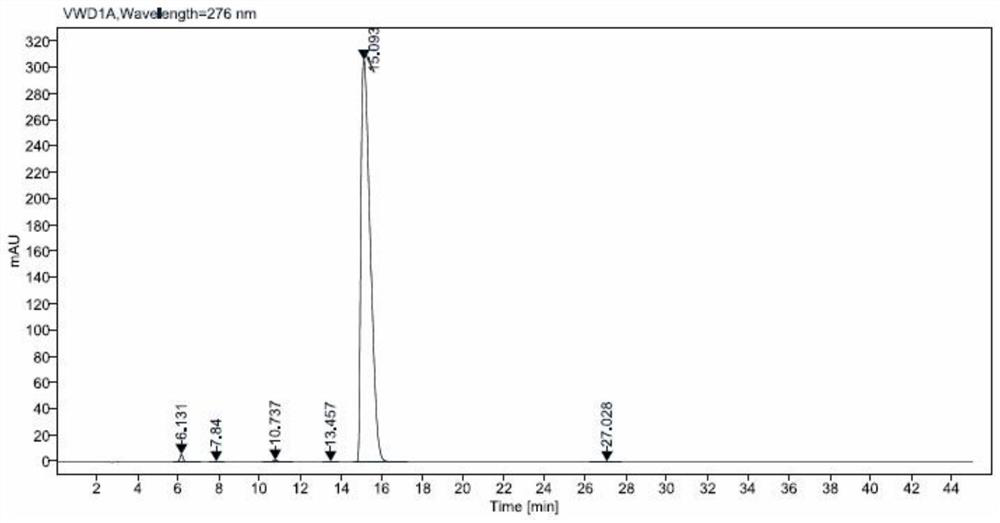
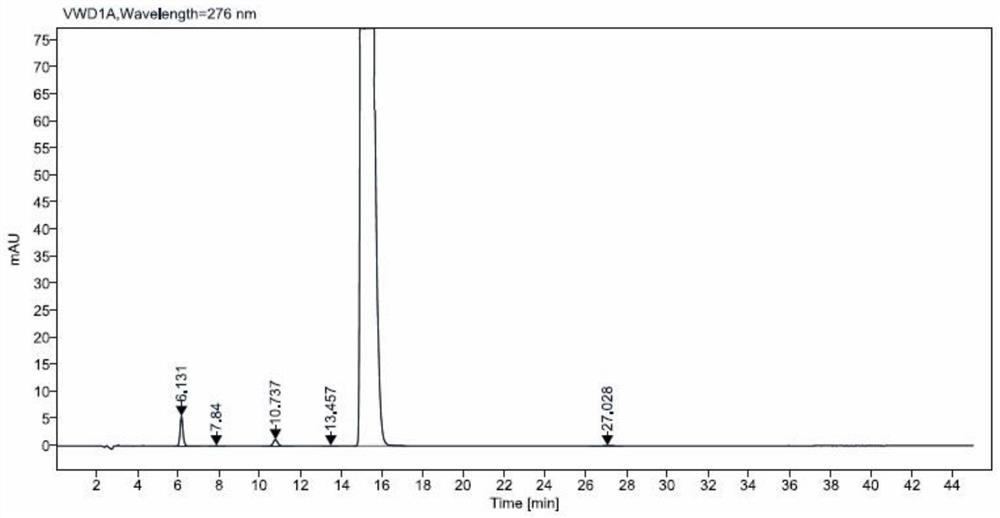
Method for analyzing related substances of terbutaline sulfate via high performance liquid chromatography
ActiveCN110057932AImprove detection efficiencyHigh sensitivityComponent separationGradient elutionHigh-performance Liquid Chromatography-UV
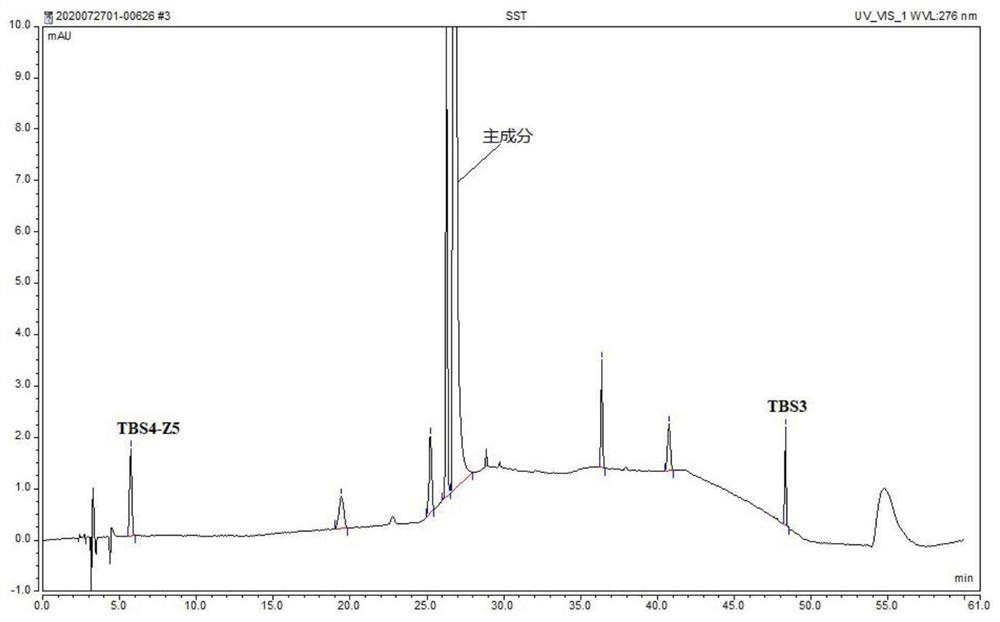
The invention provides a method for analyzing related substances of terbutaline sulfate via high performance liquid chromatography. A C18 reversed chromatographic column is used, a mobile phase is a mixture of a 0.05 M ammonium acetate buffer solution with pH 4.0 and methanol, gradient elution is carried out, and qualitative and quantitative detection of terbutaline and 9 impurities is achieved byonce sample injection. Compared with the prior art, the method provided by the invention has the advantages of being able to not only detect more impurities, but also having good sensitivity to various impurities. In the specific application, according to the detection result of each batch of samples, the limit of the key impurity formulated, and the economic applicability in the actual production process is greatly improved.
Owner:SHANGHAI XUDONG HAIPU PHARMA
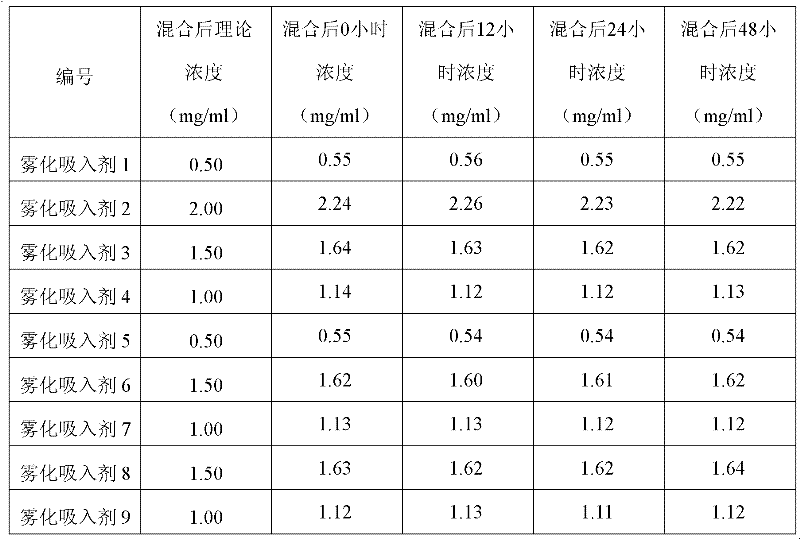
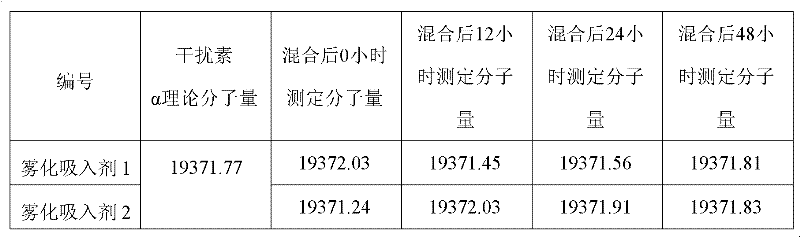
Aerosol inhalant containing interferon alpha and terbutaline sulfate
ActiveCN102416167AGood treatment effectOrganic active ingredientsPeptide/protein ingredientsAntiviral drugCurative effect
The invention belongs to the field of medicinal compositions for resisting virus, and relates to an aerosol inhalant containing interferon alpha and terbutaline sulfate. The aerosol inhalant contains a therapeutically effective amount of interferon alpha, a therapeutically effective amount of terbutaline sulfate and an appropriate amount of pharmaceutic auxiliary materials; preferably, single dose of the aerosol inhalant contains 2.5 to 30 mu g of interferon alpha, 0.5 to 2 mg of terbutaline sulfate, and an appropriate amount of pharmaceutic auxiliary materials; and more preferably, single dose of the aerosol inhalant contains 10 to 20 mu g of interferon alpha, 1 to 1.5mg of terbutaline sulfate and an appropriate amount of pharmaceutic auxiliary materials. Compared with the interferon alpha or the terbutaline sulfate, the aerosol inhalant containing the interferon alpha and the terbutaline sulfate has the advantages that the effect of treating viral pneumonia can be improved obviously.
Owner:BEIJING TRI PRIME GENE PHARMA CO LTD
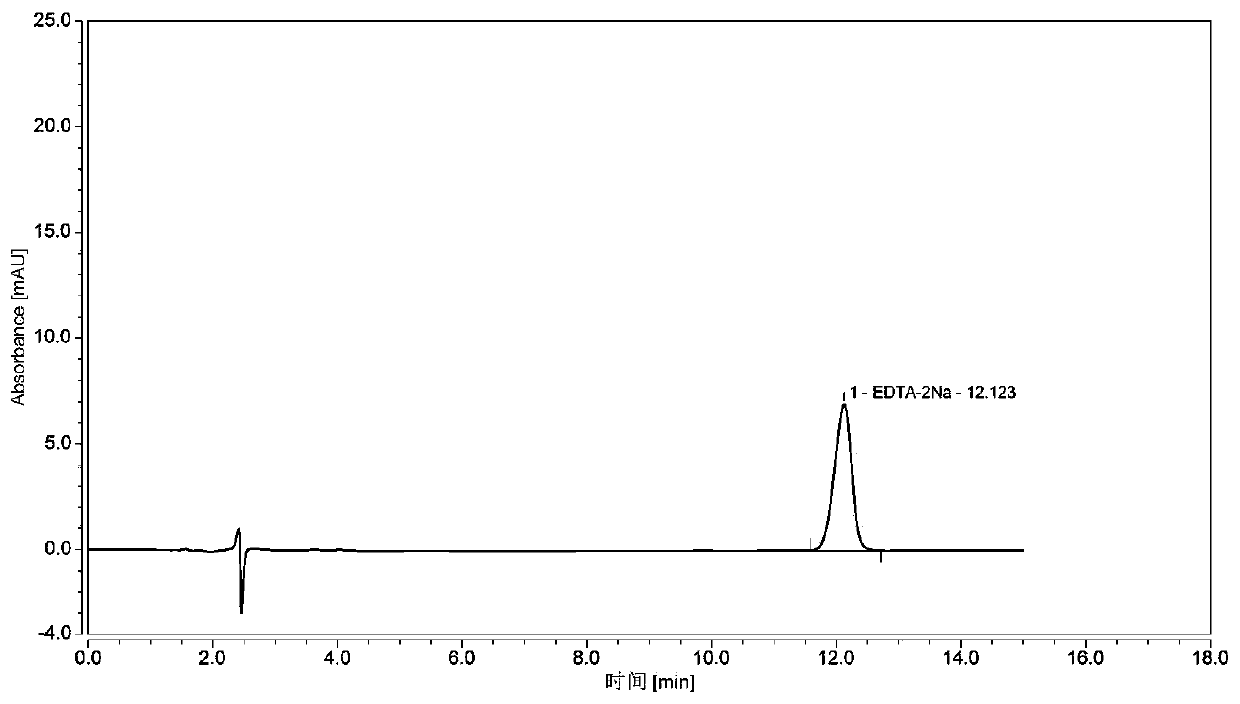
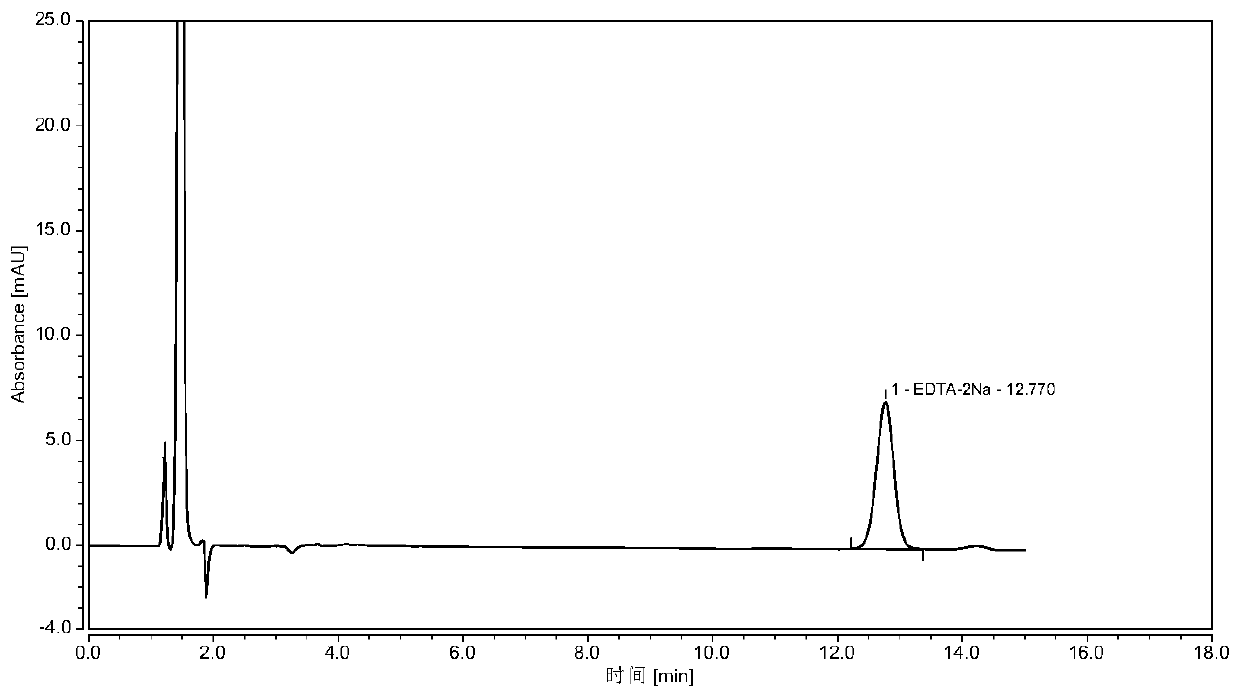
Determining method for EDTA-2Na content in terbutaline sulfate atomized solution
InactiveCN110118842AStable and uniform qualityAccurate measurementComponent separationColumn temperatureWavelength
The method of a high-precision method for EDTA-2Na content in an terbutaline sulfate atomized solution. The method comprises: (1) using sample reference; (2) using sample performance; (3) using high performance liquid chromatography to determine the Different Concentration of the standard reference solutions and the sample solutions, to be more specific, a mobile phase comprises acetonitrile and a0.006 M tetrabutyl ammonium hydroxide aqueous solution in the ratio of 1:4, then isocratic elution is carried out, a column temperature is 30 Degree Celsius, a flow rate is 1.0 mL / min, a detectionwavelength is 254 nm, injection amount is 25 MuL, and a chromatographic column is a C18 column with the inner diameter of 4.6 mm, column height of 150 mm and packing pore diameter of And um recordingthe concentrations and corresponding peak areas of the standard reference o Lutions and peak area of the sample solutions; (4) using the external standard method to determine The corresponding EDTA-2Na content according to the peak area of the sample solutions.
Owner:南京华盖制药有限公司
Preparation method of terbutaline sulfate
ActiveCN107513023APrevent oxidationHigh purityOrganic compound preparationAmino-hyroxy compound preparationInorganic saltsFiltration
Owner:SHIJIAZHUANG NO 4 PHARMA
Preparation method of terbutaline sulfate solution for inhalation
The invention relates to a preparation method of a terbutaline sulfate solution for inhalation. The terbutaline sulfate solution contains a main ingredient, a stabilizer, an iso-osmolar agent, a pH regulator and injection water, and pH value of the terbutaline sulfate solution is 3.0-5.0. The terbutaline sulfate solution has good effect on improving lung functions.
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
Method for detecting related substances in terbutaline sulfate
The invention provides a method for detecting related substances in terbutaline sulfate. According to the method, high performance liquid chromatography is adopted for detection. The method can effectively realizethe retention of the strong-polarity impurities and the effective elution of the weak-polarity impurities, and meanwhile, can reduce the damage of the solvent to a chromatographic facility.
Owner:CHENGDU BRILLIANT PHARMA CO LTD +1
Novel preparation method of terbutaline sulfate
InactiveCN110835306AMild reaction conditionsSuitable for industrial scale-upOrganic compound preparationCarboxylic acid esters preparationBronchial SpasmDisease
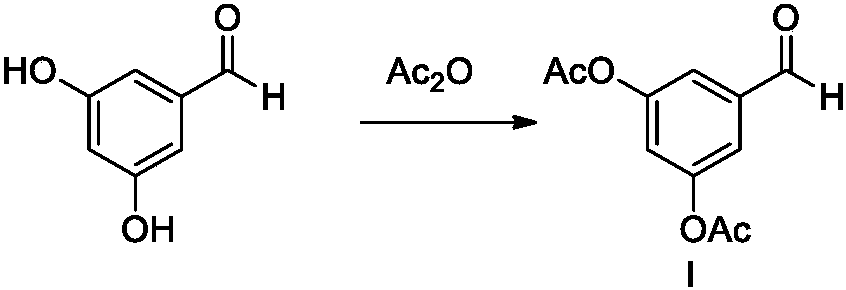
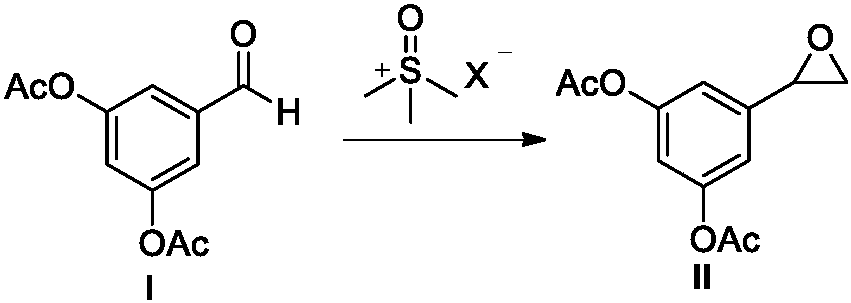
The invention belongs to the field of organic synthesis of medicines, and concretely relates to a novel preparation method of a medicine terbutaline sulfate for treating bronchial spasm caused by bronchial asthma, chronic bronchitis, emphysema and other lung diseases. The synthesis route of the preparation method comprises the following steps: reacting 3,5-dihydroxybenzaldehyde with acetic anhydride to generate a compound I; reacting the compound I with trimethyloxosulfonium halide to generate a compound II; reacting the compound II with tert-butylamine to generate terbutaline; and salifying the terbutaline to generate the terbutaline sulfate. The method has the advantages of avoiding of dangerous chemical reagents, low price of adopted reagents, mild reaction conditions, and suitablenessfor industrial amplification.
Owner:HARVEST PHARMA HUNAN CO LTD
Solution preparation for terbutaline sulphate aerosol inhalation and preparation method of solution preparation
InactiveCN110693861AAvoid first pass effectAvoid destructionOrganic active ingredientsPowder deliveryMedicinal chemistryPharmacology
The invention provides a solution preparation for terbutaline sulphate aerosol inhalation and a preparation method of the solution preparation. The solution preparation for terbutaline sulphate aerosol inhalation comprises terbutaline sulphate and / or a hydrate thereof, an osmotic pressure regulator, a pH regulator and a solvent. The solution preparation for terbutaline sulphate aerosol inhalationdisclosed by the invention has the characteristics of being efficient, low in toxicity, good in stability and high in safety degree.
Owner:BEIJING INCREASE INNOVATIVE DRUG RESEARCH CO LTD
A processing method for improving the stability of terbutaline sulfate injection
ActiveCN108078918BImprove stabilityReduce adverse effectsOrganic active ingredientsInorganic non-active ingredientsPhysical chemistrySS - Stainless steel
Owner:石药银湖制药有限公司
Method for preparing terbutaline sulphate crystal B fulfilling medicinal requirements
InactiveCN101391965AChemically stableStable physical propertiesOrganic compound preparationRespiratory disorderDissolutionSolvent
The invention provides a method for preparing terbutaline sulfate crystal form B, which comprises the steps: a solvent is selected and 1-(3, 5 dibenzyloxyphenyl)-2-t-butylamido ethanol is dissolved in the solvent, the phenmethyl is removed by hydrogenolysis of a universal method to obtain terbutaline free alkali, and then sulphuric acid is added to form the terbutaline sulfate of crystal form B; or the terbutaline sulfate of non-crystal form B is dissolved in the solvent and stirred at certain temperature until the terbutaline sulfate of the non-crystal form B is converted to form the terbutaline sulfate crystal form B by the dissolution and precipitation process, then the precipitated terbutaline sulfate crystal form B is recycled. The invention has the advantages that the terbutaline sulfate crystal form B which has stable chemical and physical appearances and meets the requirements of pharmaceutical preparations is obtained by the simple method.
Owner:李勤耕
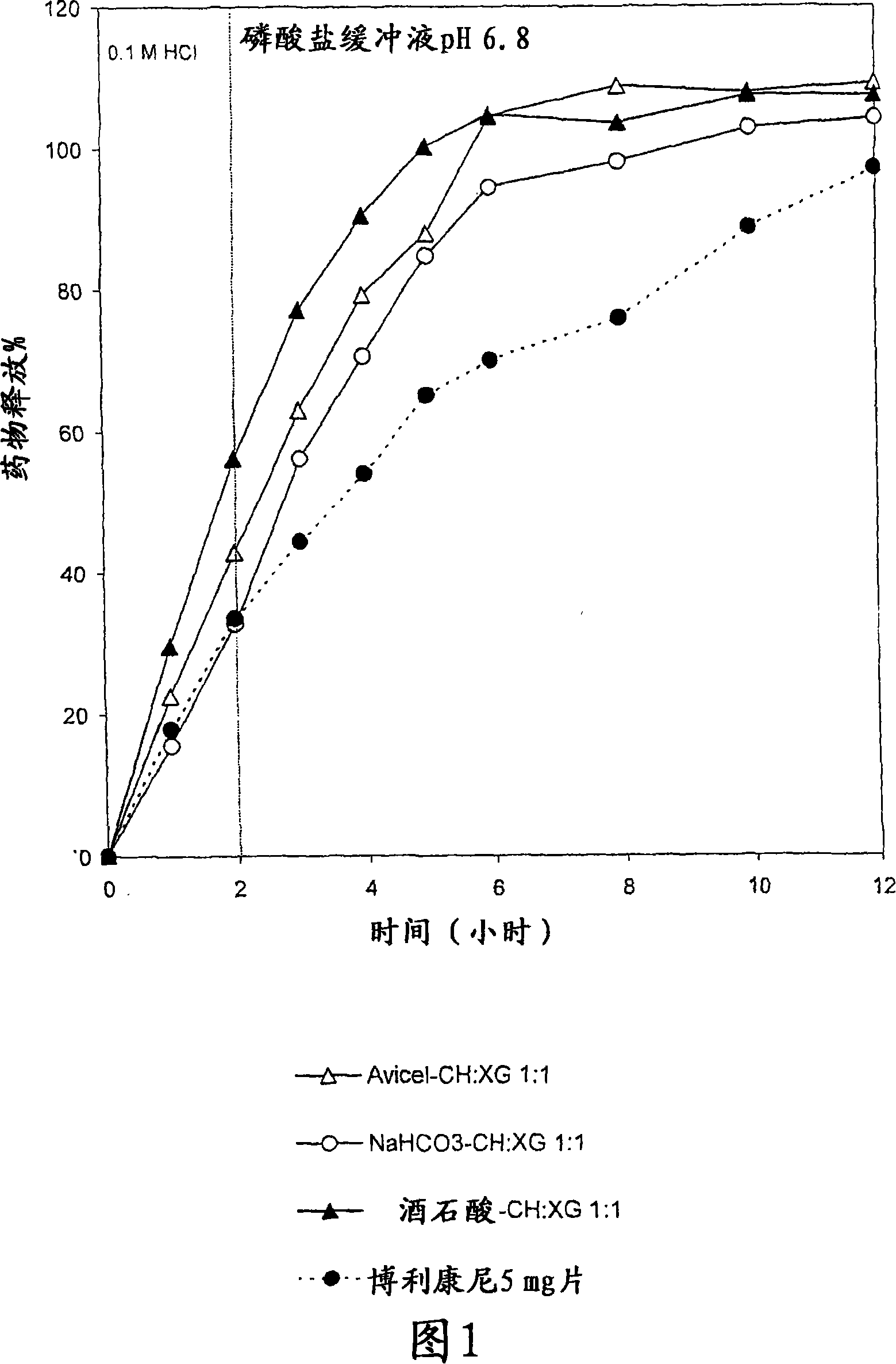
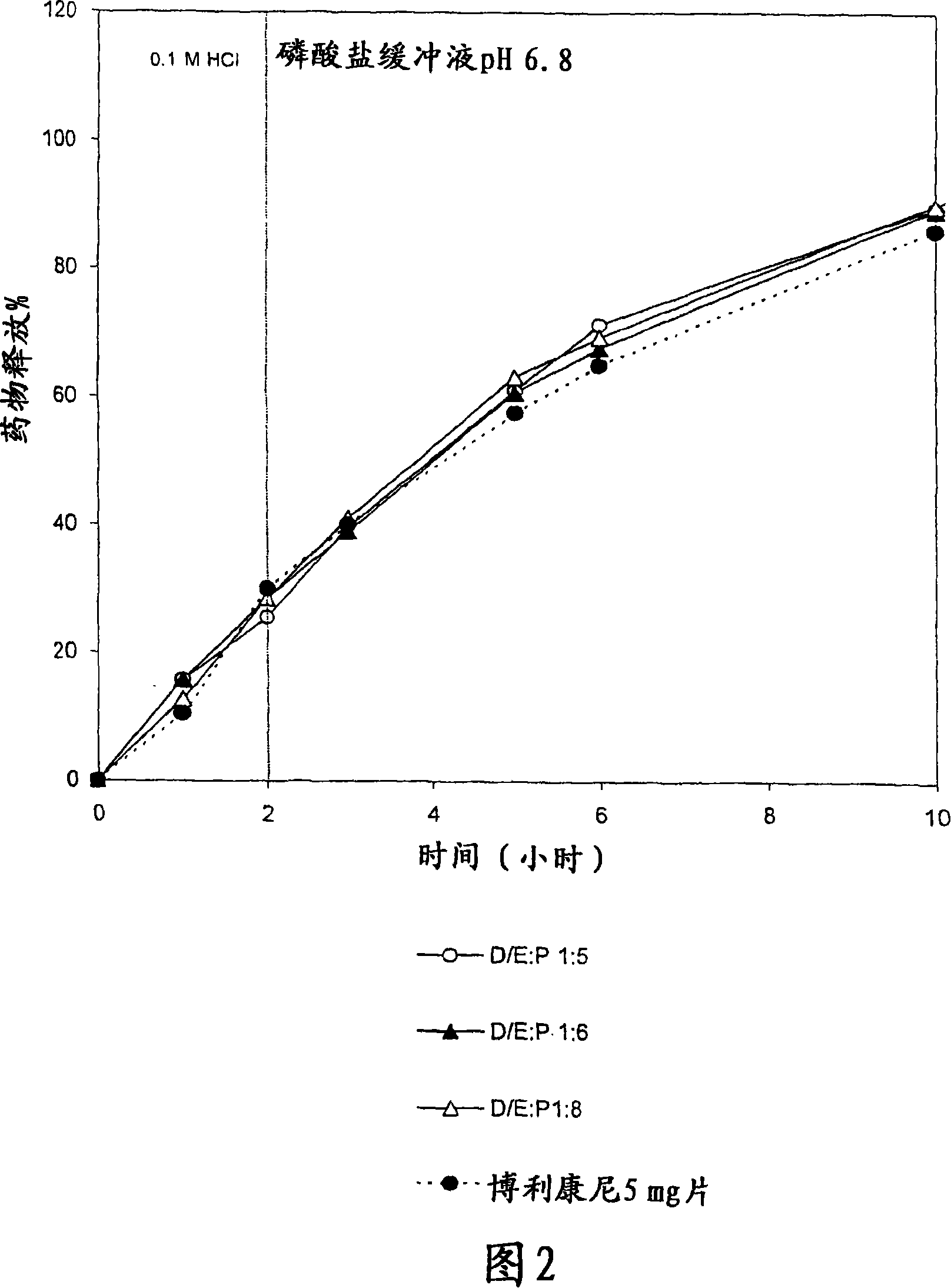
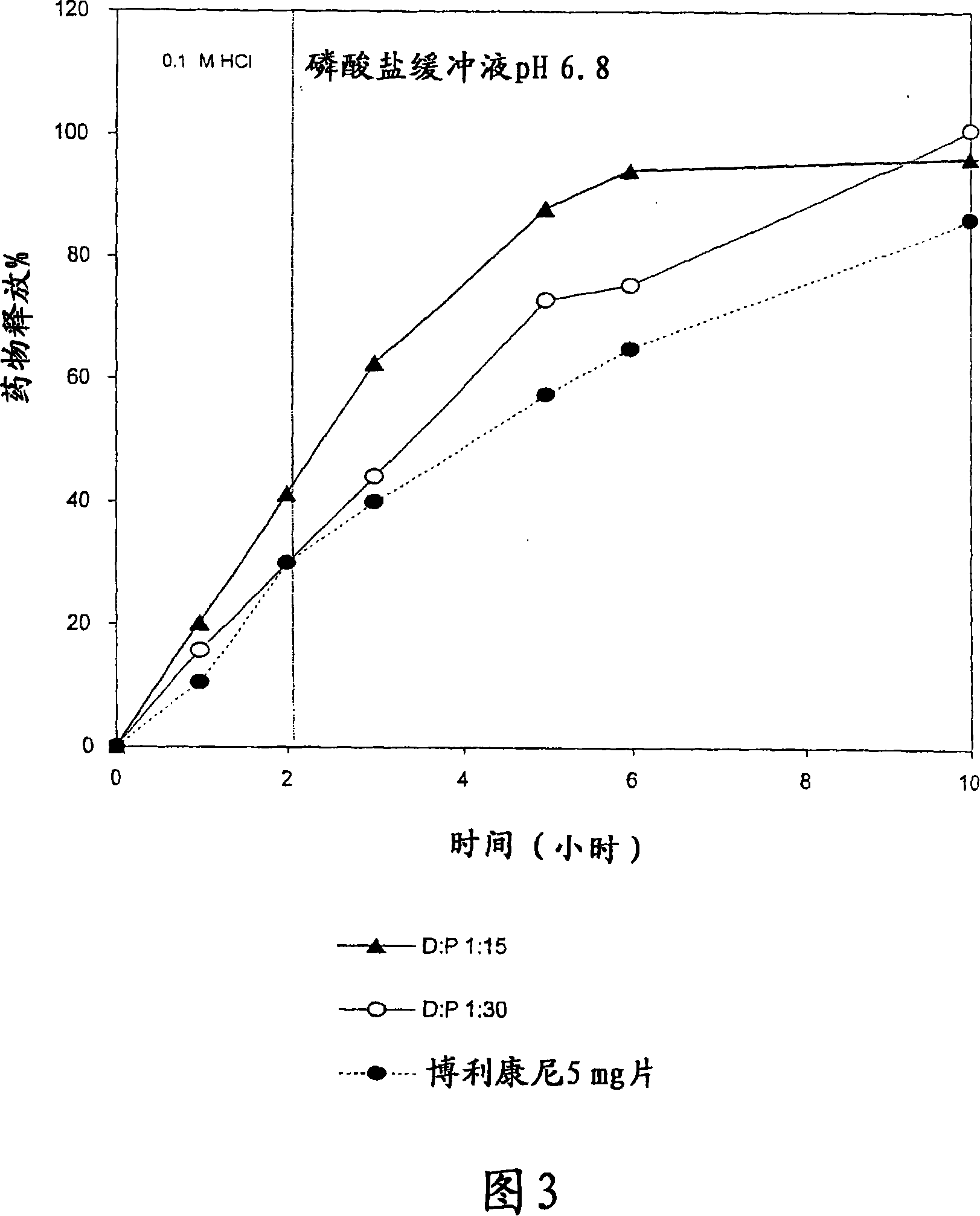
Pharmaceutical polymer composition for oral controlled-release delivery of terbutaline sulfate
The present invention is directed to a controlled-release pharmaceutical composition providing a sustained delivery of the basic drug Terbutaline sulfate, said composition comprising at least Terbutaline sulfate or a derivative thereof as an active agent, and further comprising an inactive matrix, said matrix comprising a hydrophilic polysaccharide polymer mixture, said mixture comprising chitosan or a derivative thereof, and further comprising xanthan gum or a derivative thereof, wherein the ratio of xanthan gum and chitosan within said mixture is in the range from about 1:10 to about 10:1, and said composition optionally comprising sodium bicarbonate.
Owner:约旦制药公司
Terbutaline sulfate dropping pill and its preparing method
InactiveCN1813695ARapid dissolutionQuick effectOrganic active ingredientsPill deliveryMedicineDrug product
The present invention discloses a medicine terbutaline sulfate dripping pills preparation with the action of relieving asthma and its preparation process. It is made up by using terbutaline sulfate and matrix for making dripping pills.
Owner:陈茜
Oxygen-driven atomized inhalation solution for infant asthmatic suffocating pneumonia
InactiveCN102114027ARelieves spasmodic contractionsInhibition releasePharmaceutical delivery mechanismAntiviralsDiseaseSide effect
The invention belongs to the medical technology field, in particular discloses an oxygen-driven atomized inhalation solution for infant asthmatic suffocating pneumonia. The atomized inhalation solution is prepared by using budesonide, terbutaline sulfate, ambroxol hydrochloride, aminophylline and ribavirin as raw materials, respectively and proportionally compounding according to different characteristics of the medicaments, uniformly mixing, packaging into 5 ml / injection, sealing, sterilizing and labeling. The invention has the advantages of effectively relieving the symptoms of the infant asthmatic suffocating pneumonia, increasing the curative rate, shortening the course of disease, lessening adverse reactions, being convenient and safe to use, and the like. Tested by 150 clinical cases of infants with asthmatic suffocating pneumonia, the total curative rate reaches 92 percent without any special toxic or side effect.
Owner:李运智
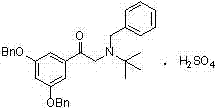
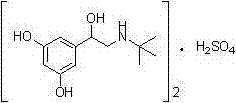
Terbutaline sulfate intermediate, preparation method thereof, and method for preparing terbutaline sulfate from terbutaline sulfate intermediate
ActiveCN111499528AStable in natureReduce usageOrganic compound preparationCarboxylic acid esters preparationHydrobromideCombinatorial chemistry
The invention discloses a terbutaline sulfate intermediate, a preparation method thereof, and a method for preparing terbutaline sulfate from the terbutaline sulfate intermediate, and belongs to the technical field of medical chemistry. The novel method for preparing terbutaline sulfate is mild in reaction condition, high in yield and environment-friendly, and comprises the following steps: firstly, providing the terbutaline sulfate intermediate represented by formula I; and then carrying out hydrogenation reduction by taking 10% Pd / C as a catalyst and an alcohol-water solution as a solvent toobtain the terbutaline sulfate represented by formula II. The free alkali hydrochloride or hydrobromide in a formula I is converted into the compound of the formula I before hydrogenation reduction,and the terbutaline sulfate is directly obtained after hydrogenation reduction, so extremely unstable terbutaline free alkali is prevented from appearing independently, and the method has the advantages of mild reaction conditions, high product yield, high purity, low production cost and the like, and is beneficial to industrial production of the terbutaline sulfate.
Owner:成都瑞特恩科技有限公司
Preparation method of pharmaceutical terbutaline sulfate crystal form B
InactiveCN109988074AMild reaction conditionsSafe and simple preparation processOrganic compound preparationOrganic chemistry methodsAlcoholKetone solvents
The invention discloses a preparation method of a pharmaceutical terbutaline sulfate crystal form B. The preparation method comprises the following steps: S1, dissolving terbutaline sulfate of an amorphous form B in pure water, and conducting stirring and dissolving to obtain a terbutaline sulfate aqueous solution; S2, dropwise adding the amorphous form B terbutaline sulfate aqueous solution obtained in the step S1 into an alcohol or ketone solvent, conducting stirring for dissolving, adding a crystal seed of the terbutaline sulfate crystal form B, and conducting stirring for crystallizing toobtain a terbutaline sulfate crystal solution of the crystal form B; and S3, filtering the terbutaline sulfate crystal liquid with the crystal form B obtained in the step S2 and conducting drying to obtain a terbutaline sulfate crystal form B, wherein the ratio of the mass (unit: g) of the amorphous form B terbutaline sulfate to the mass (unit: g) of pure water to the mass (unit: g) of the crystalseed of the terbutaline crystal form B to the volume (unit: mL) of the alcohol or ketone solvent is 1:(4-5):(0.01-0.1):(50-60). The method has the advantages of simple operation, high repeatability,environment-friendly raw materials, and easy realization of industrial production application.
Owner:YAOPU SHANGHAI PHARMA TECH CO LTD
Terbutaline sulfate oral instant film and preparation method thereof
InactiveCN102961365BPleasant tasteImprove palatabilityOrganic active ingredientsPharmaceutical non-active ingredientsSevere disabilityBULK ACTIVE INGREDIENT
The invention discloses a terbutaline sulfate oral instant film and a preparation method thereof, and belongs to the technical field of medicines. The terbutaline sulfate oral instant film comprises active ingredients, such as terbutaline sulfate, film-forming materials, disintegrating agents and opacifying agent with effective dosages, and necessary pharmaceutically acceptable filling agents, sweetening agents and wetting agents; and coloring agents are added according to requirements. The terbutaline sulfate oral instant film is good in palatability, fast in response, small in size, and suitable for children, elders, completely-bedridden persons and patients with severe disability; the film can be rapidly dissolved in a disintegrating mode in the oral cavity; water is not needed to orally take; and people can take the medicines any time under a condition that water is inconvenient to drink.
Owner:天津市聚星康华医药科技有限公司
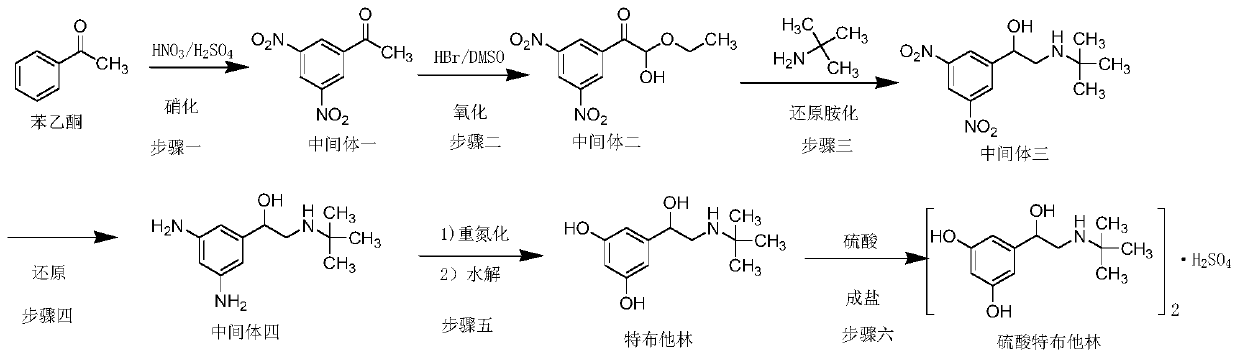
Preparation method of terbutaline sulfate
ActiveCN111454164AEasy to purifyShorten the total synthetic routeOrganic compound preparationAmino-hyroxy compound preparationAcetophenoneActive ingredient
The invention relates to the field of preparation of chemicals, in particular to a preparation method of terbutaline sulfate. The invention provides a preparation method of terbutaline sulfate. With simple and low-cost acetophenone as an initial raw material, the terbutaline is prepared through five-step reaction; then the terbutaline is subjected to salifying and purification to obtain terbutaline sulfate. According to the method disclosed by the invention, the total synthesis route of terbutaline is effectively shortened, so that the method is simple in intermediate purification, single in reaction solvent, simple in process, mild in reaction condition, easy to operate, high in total yield and more suitable for industrial production; the burden of workshop waste liquid treatment and purification is relieved, the three wastes and reaction energy consumption are reduced, the whole route is combined, research and control of raw material medicine impurities are better facilitated, and working hours are shortened technically and the three wastes and reaction energy consumption are reduced technically.
Owner:SHANDONG MEITAI PHARMA CO LTD
Aerosol inhalation medicine composition for treating cough and asthma and preparation method thereof
InactiveCN109674847AOrganic active ingredientsDispersion deliveryTraditional medicineTerbutaline Sulfate
The invention belongs to the technical field of medicine, and provides an aerosol inhalation medicine composition for treating cough and asthma. The aerosol inhalation medicine composition for treating cough and asthma is prepared from 300 to 600ug of budesonide, 600 to 1200ug of terbutaline sulfate, 10 to 15mg of ambroxol hydrochloride, 0.5 to 1mL of radix astragali extracts, 0.5ml of propanedioland 10ml of normal saline. The invention also provides a preparation method of the aerosol inhalation medicine composition.
Owner:SHANDONG PROVINCIAL HOSPITAL
Preparation method of terbutaline sulfate and B crystal form thereof
PendingCN112250586AReduce usageShort reaction pathOrganic compound preparationOrganic chemistry methodsPtru catalystPhenyl group
The invention relates to a preparation method of terbutaline sulfate and a B crystal form thereof, which comprises the following steps: sequentially carrying out bromination, reduction reaction and substitution reaction to obtain 1-[3, 5-bis (benzyloxy) phenyl]-2-(tert-butylamino) ethanol; reacting 1-[3, 5-di (benzyloxy) phenyl]-2-(tert-butylamino) ethanol with a reducing agent and a catalyst, performing salifying with sulfuric acid to obtain a terbutaline sulfate crude product, and crystallizing the terbutaline sulfate crude product under a heating reflux condition to obtain the terbutaline sulfate medicinal B crystal form. Raw materials and auxiliary materials used in the method are cheap and easy to obtain, highly toxic and explosive reagents are avoided in the reaction process, the whole reaction route is short, operation is easy and convenient, reaction conditions are mild and safe, the yield of the obtained finished product terbutaline sulfate and the medicinal B crystal form thereof is high, the yield of terbutaline sulfate is 83% or above, and the yield of the medicinal B crystal form is 70% or above, and industrialized production can be achieved easily.
Owner:NINGBO TEAM PHARMA
Method for detecting terbutaline sulfate injection and related substances
ActiveCN114674951AIncreased durabilityEasy to separateComponent separationAgainst vector-borne diseasesBiologyAmmonium formate
The invention relates to the technical field of drug detection methods, in particular to a detection method for determining terbutaline sulfate injection and related substances, the detection method adopts high performance liquid chromatography for detection, and the detection conditions are as follows: a mobile phase is composed of a mobile phase A and a mobile phase B, wherein the mobile phase A is an ammonium formate-formic acid buffer solution containing 3.75-4.31 g / L of sodium hexanesulfonate and 1.25-1.57 g / L of sodium sulfate, and the pH value is 3.0 + / -0.1; the mobile phase B is methanol; the mixing volume ratio of the mobile phase A to the mobile phase B is 80: 20; the detection method can realize simultaneous detection of degradation impurities of raw material medicines and preparations, and has the advantages of high separation degree, good durability and the like.
Owner:广东金城金素制药有限公司
A kind of preparation method of terbutaline sulfate
ActiveCN107513023BPrevent oxidationHigh purityOrganic compound preparationAmino-hyroxy compound preparationInorganic saltsPtru catalyst
The invention relates to a preparation method of terbutaline sulfate, which comprises the following steps: dissolving a compound of formula I or formula II into a solvent A, adding a metal catalyst for catalytic hydrogenation, so as to obtain 5-[2-[(1,1-dimethylethyl)amido]-1-ethoxyl]-1,3-resorcinol; after the reaction is finished, filtering, collecting filter liquor, adding a certain amount of concentrated sulfuric acid in the filter liquor, after stirring is stopped, recycling the solvent A, adding a solvent B into a residue, stirring to separate out a white crystal substance, performing suction filtration, thus obtaining the terbutaline sulfate. According to the method provided by the invention, the treating difficulty after the reaction is greatly reduced, the introduction of water and inorganic salt is avoided, and the product content is high; furthermore, the stability of the terbutaline sulfate is obviously improved by distilling the product under acidic conditions, the catalyst and the reaction solvents are recyclable, so that the environmental protection pressure is greatly reduced.
Owner:SHIJIAZHUANG NO 4 PHARMA
Preparation method of terbutaline sulfate
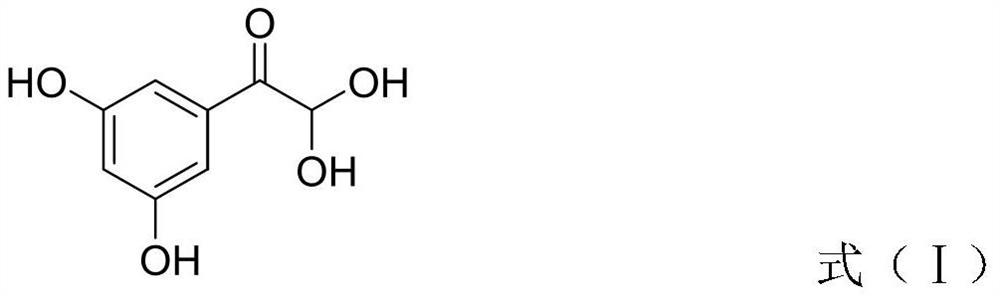
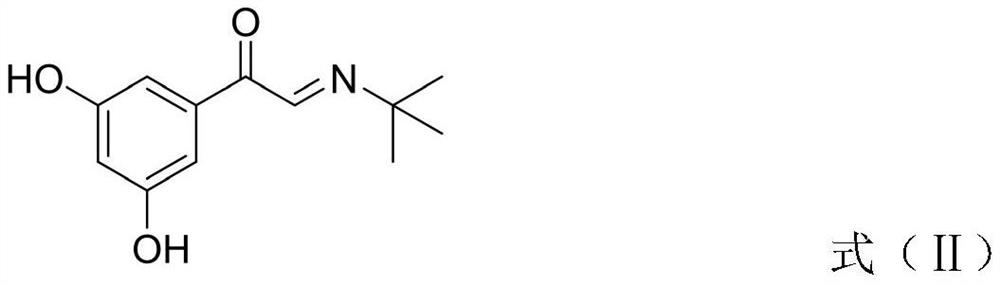
InactiveCN113045437AShort reaction stepsGood atomic economicsOrganic compound preparationCarbonyl compound preparation by oxidationAcetophenoneEnvironmental chemistry
The invention provides a preparation method of terbutaline sulfate. The preparation method comprises the following steps: with 3,5-dihydroxyacetophenone as a raw material, directly carrying out an oxidation reaction on 3,5-dihydroxyacetophenone without protecting a phenolic hydroxyl group so as to obtain aromatic glyoxal, and then carrying out a condensation reaction, a reduction reaction and sulfuric acid salification on the aromatic glyoxal and tert-butylamine to obtain terbutaline sulfate. According to the method, a high-risk operation unit for hydrogenation debenzylation in an existing terbutaline sulfate preparation process is eliminated; and meanwhile, bromination reaction with relatively large pollution to the environment is avoided, and the whole process is more suitable for industrial production.
Owner:北京睿悦生物医药科技有限公司 +1
Synthesis method of terbutaline and application of terbutaline in preparation of terbutaline sulfate
ActiveCN112209841AAvoid the process of hydrodebenzylationReduce pollutionOrganic compound preparationCarbonyl compound preparationAcetophenoneHydrolysis
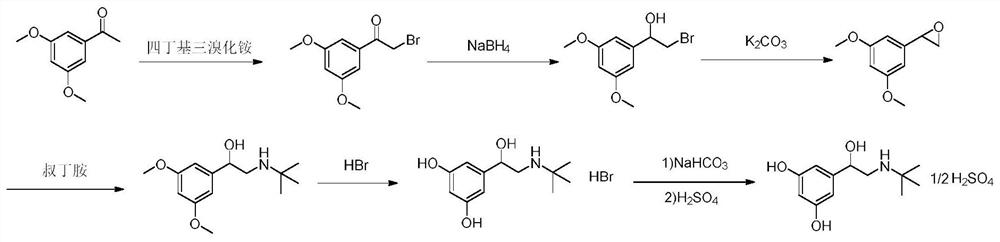
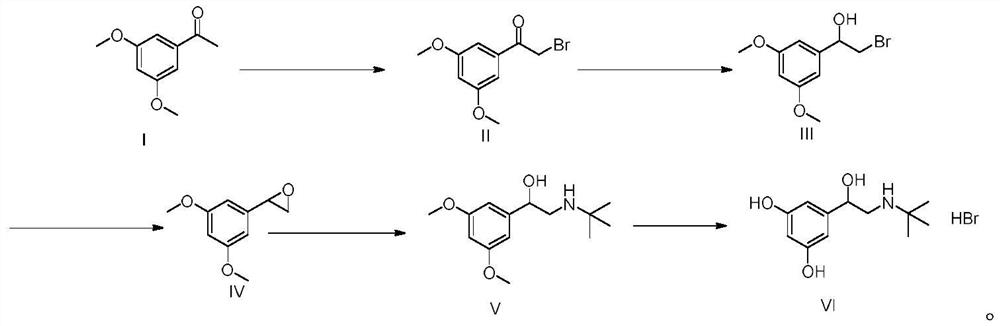
The invention discloses a synthesis method of terbutaline, which is characterized by comprising the following steps: (1) reacting 3, 5-dimethoxyacetophenone with tetrabutylammonium tribromide to obtain 2-bromo-1-(3, 5-dimethoxyphenyl)ethanone; (2) reacting the 2-bromo-1-(3, 5-dimethoxyphenyl)ethanol with a reducing agent in methanol to obtain 2-bromo-1-(3, 5-dimethoxyphenyl)ethanol; (3) carrying out cyclization reaction on the 2-bromo-1-(3, 5-dimethoxyphenyl)ethanol under an alkaline condition to generate 2-(3, 5-dimethyloxy) ethylene oxide; (4) carrying out a reaction on the 2-(3, 5-dimethyloxy) ethylene oxide and tert-butylamine to generate 1-(3, 5-dimethoxyphenyl)-2-tert-butylamino-ethanol; and (5) hydrolyzing under an acidic condition to obtain a salt of 1-(3, 5-dihydroxyphenyl)-2-tert-butylamino-ethanol, namely terbutaline. The total molar yield is about 60% and is far higher than the yield in the prior art, and the synthesis method of terbutaline and the application of terbutaline in the preparation of terbutaline sulfate are suitable for industrial production.
Owner:YANGZHOU ZHONGBAO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com