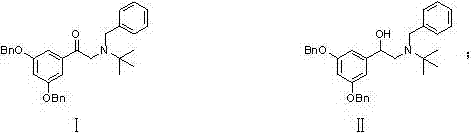Preparation method of terbutaline sulfate
A technology of terbutaline sulfate and concentrated sulfuric acid, which is applied in the field of medicine and chemical industry, can solve the problems of increased post-processing cost and environmental protection pressure of mixed solvents, high market price of raw materials, cumbersome post-processing, etc., to avoid high energy consumption operating conditions The effect of using, avoiding the introduction of water and inorganic salts, and reducing the difficulty of post-reaction treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
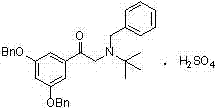
[0042]Add 1.18kg of the compound of formula I to 13.8L of glacial acetic acid and 59g of palladium carbon, replace with nitrogen 6 times, hydrogen 6 times, and stir at 60°C under normal pressure for 5 hours in the dark. Cool down to 10-15°C, suction filter under nitrogen atmosphere, place the suction filtrate in a reaction flask, add acetic acid solution of concentrated sulfuric acid equivalent to 0.5eq of the compound of formula I (mass volume concentration is 5%) under nitrogen protection, stir for 15min, Transfer to a distillation flask, replace with nitrogen, distill under reduced pressure, concentrate, cool down to room temperature, add 650ml of methanol, stir and crystallize under nitrogen atmosphere for 30min, drop to 10-15℃ for 1.5h, suction filter to obtain a white crystalline solid 645g, yield 98.4%, purity 99.87%.
Embodiment 2
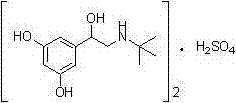
[0044] Add 1.19 kg of the compound of formula II to 17.8 L of glacial acetic acid and 119 g of Ni, replace with nitrogen 6 times and hydrogen 6 times, and stir at 50°C under normal pressure for 4 hours in the dark. Cool down to 10-15°C, suction filter under a nitrogen atmosphere, place the suction filtrate in a reaction flask, add an acetic acid solution of concentrated sulfuric acid equivalent to 0.505 eq of the compound of formula I (mass volume concentration: 8%) under nitrogen protection, and stir for 20 minutes. Transfer to a distillation flask, replace with nitrogen, distill under reduced pressure, concentrate, cool down to room temperature, add methanol, stir and crystallize under a nitrogen atmosphere for 30 minutes, drop to 10-15°C for 2 hours, and filter with suction to obtain 652 g of a white crystalline solid. Yield 99.1%, purity 99.83%.
Embodiment 3
[0046] Add 1.18kg formula I compound to 11.8L glacial acetic acid and 59g Pd(OH) 2 , 6 times of nitrogen replacement, 6 times of hydrogen replacement, stirring at 40° C. under normal pressure in the dark for 5 h. Cool down to 10-15°C, suction filter under nitrogen atmosphere, place the suction filtrate in a reaction flask, add acetic acid solution of concentrated sulfuric acid equivalent to 0.495eq of the compound of formula I (mass volume concentration is 5%) under nitrogen protection, and stir for 15min. Transfer to a distillation flask, replace with nitrogen, distill under reduced pressure, concentrate, cool down to room temperature, add 1300ml of methanol, stir and crystallize under nitrogen atmosphere for 30min, drop to 10-15°C for 1.5h, and suction filter to obtain a white crystalline solid 642g, yield 98.0%, purity 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com