Preparation method of terbutaline sulfate
A technology of terbutaline sulfate and terbutaline, which is applied in the preparation of organic compounds, chemical instruments and methods, preparation of nitro compounds, etc., can solve the problems of increasing operational risks, low atom utilization, and complicated routes. , to achieve the effect of shortening working hours, conducive to research and control, and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
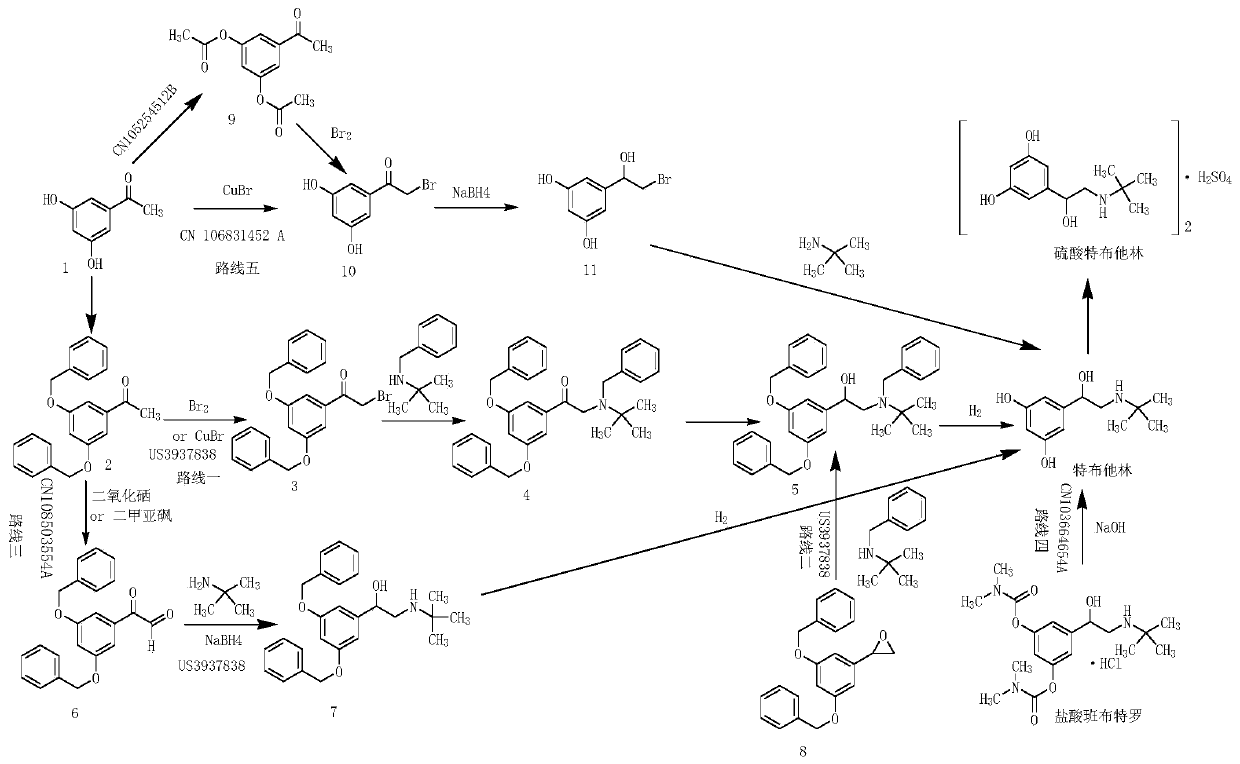
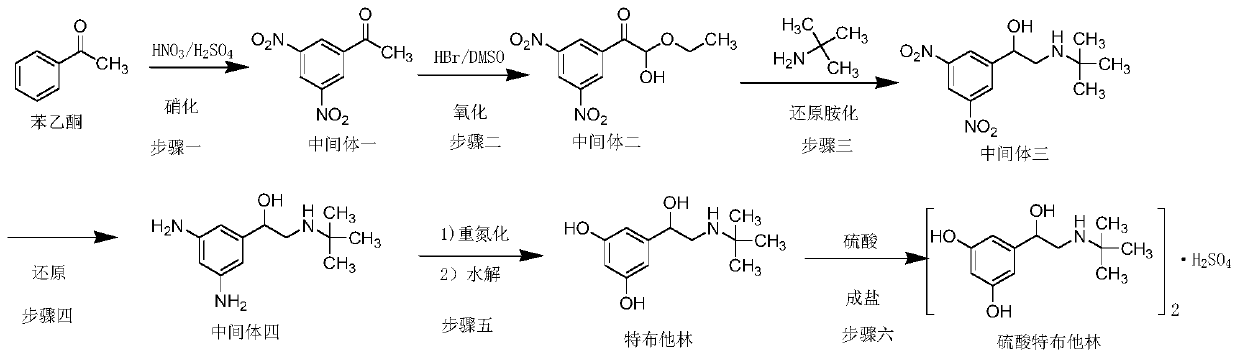
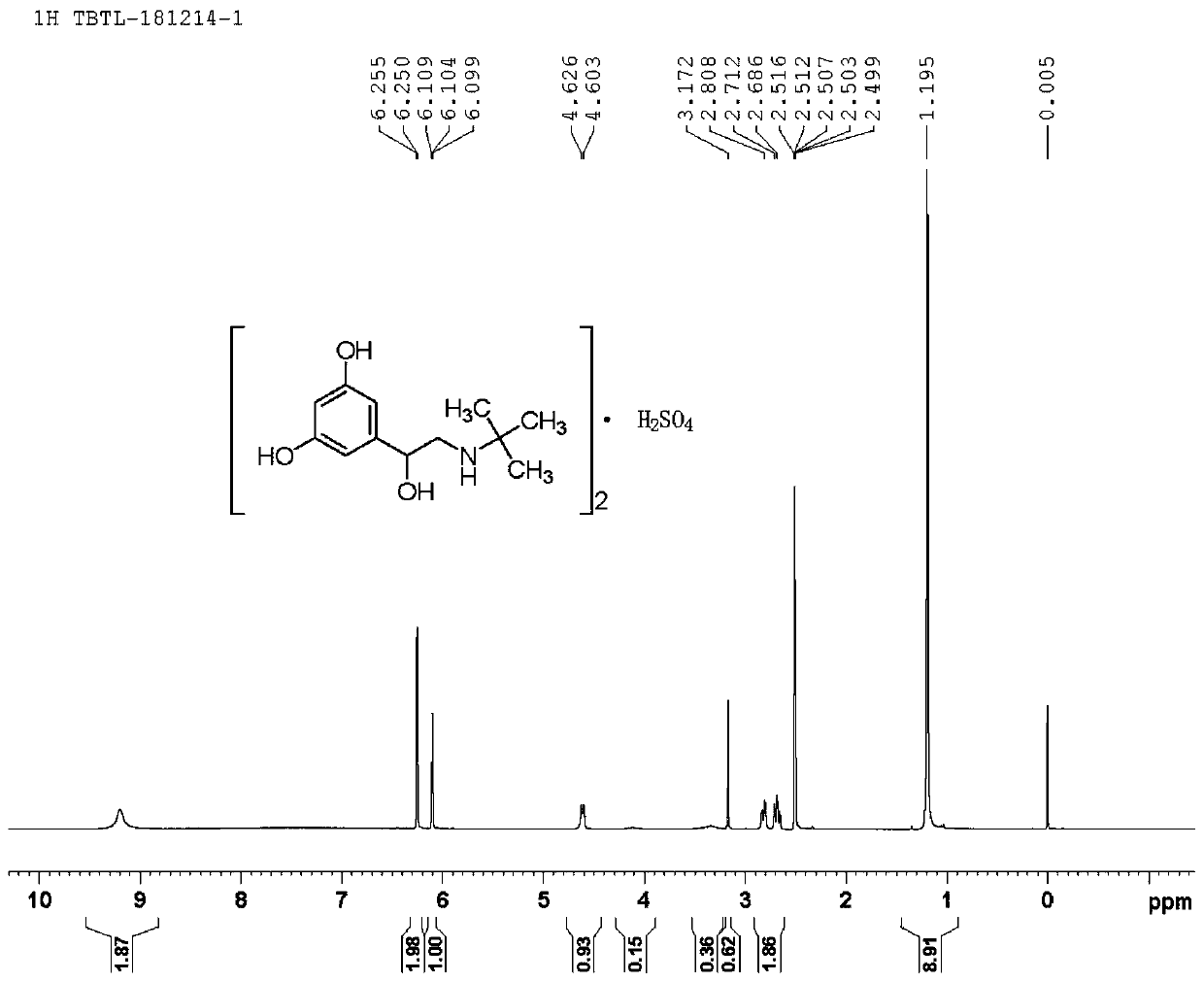
Image
Examples
Embodiment 1
[0064] The present embodiment provides a kind of preparation method of terbutaline sulfate, comprises the following steps:
[0065] Step 1, acetophenone, sulfuric acid and nitric acid are subjected to nitration reaction, and intermediate 1 is obtained after water analysis and organic solvent crystallization and purification;
[0066] The preparation of intermediate one (3,5-dinitroacetophenone):
[0067] In a 2L three-necked flask, add concentrated sulfuric acid (1200ml), cool down to 0°C, start mechanical stirring, then add acetophenone (240.00g, 2.00mol) in batches, after the addition, nitric acid (320ml, density 1.42g / ml, the mass fraction is 69.2%) drop about 20ml into it to initiate the reaction, start to heat up, the temperature rises to 60-70°C, the color of the reaction solution becomes darker, turn off the heating, slowly add the remaining nitric acid, strictly control the rate of addition, to avoid reaction Vigorously, after dropping, raise the temperature to 140°C...
Embodiment 2
[0084] The difference between this example and Example 1 lies in the preparation of the intermediate tris(2-tert-butylamino-1-(3,5-dinitrophenyl)ethanol):
[0085] Add Intermediate II (40.00g, 0.15mol), tert-butylamine (16.22g, 0.22mol), and 500ml of absolute ethanol to a 2L three-necked flask at room temperature, and raise the temperature to reflux for 4h; add sodium borohydride (8.36g, 0.22mol) in batches ), a large number of bubbles are generated, and it takes about 1.5 hours to add, continue to reflux for 1 hour, until no bubbles are released, evaporate the solvent under reduced pressure, add 500ml dichloromethane to the concentrate, wash once with 200ml 1N hydrochloric acid, separate liquid, organic Wash once with 200ml of 5% aqueous sodium hydroxide solution, separate the liquids, wash the organic phase once with 200ml saturated brine, separate the liquids, dry the organic phase over anhydrous sodium sulfate, filter with suction, concentrate, add 30ml of dichloromethane a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com