Preparation method of terbutaline sulfate
A technology of terbutaline sulfate and acid binding agent, which is applied in the field of preparation of terbutaline sulfate, can solve the problems of expensive raw materials, long reaction steps, complicated operations, etc. Mild conditions, long solution steps and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
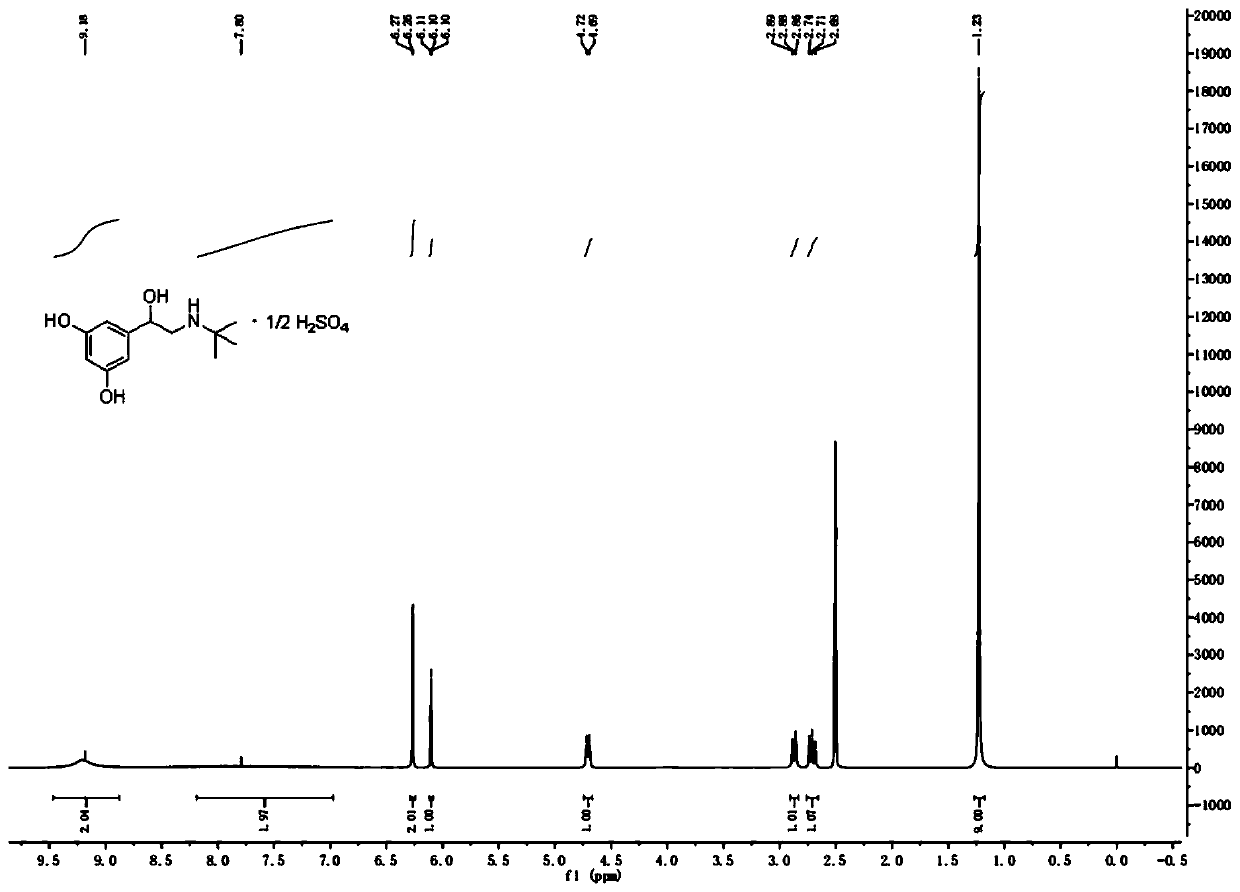
[0033] A preparation method of terbutaline sulfate, specifically comprising:
[0034] (1) Preparation of 3,5-dibenzyloxyacetophenone
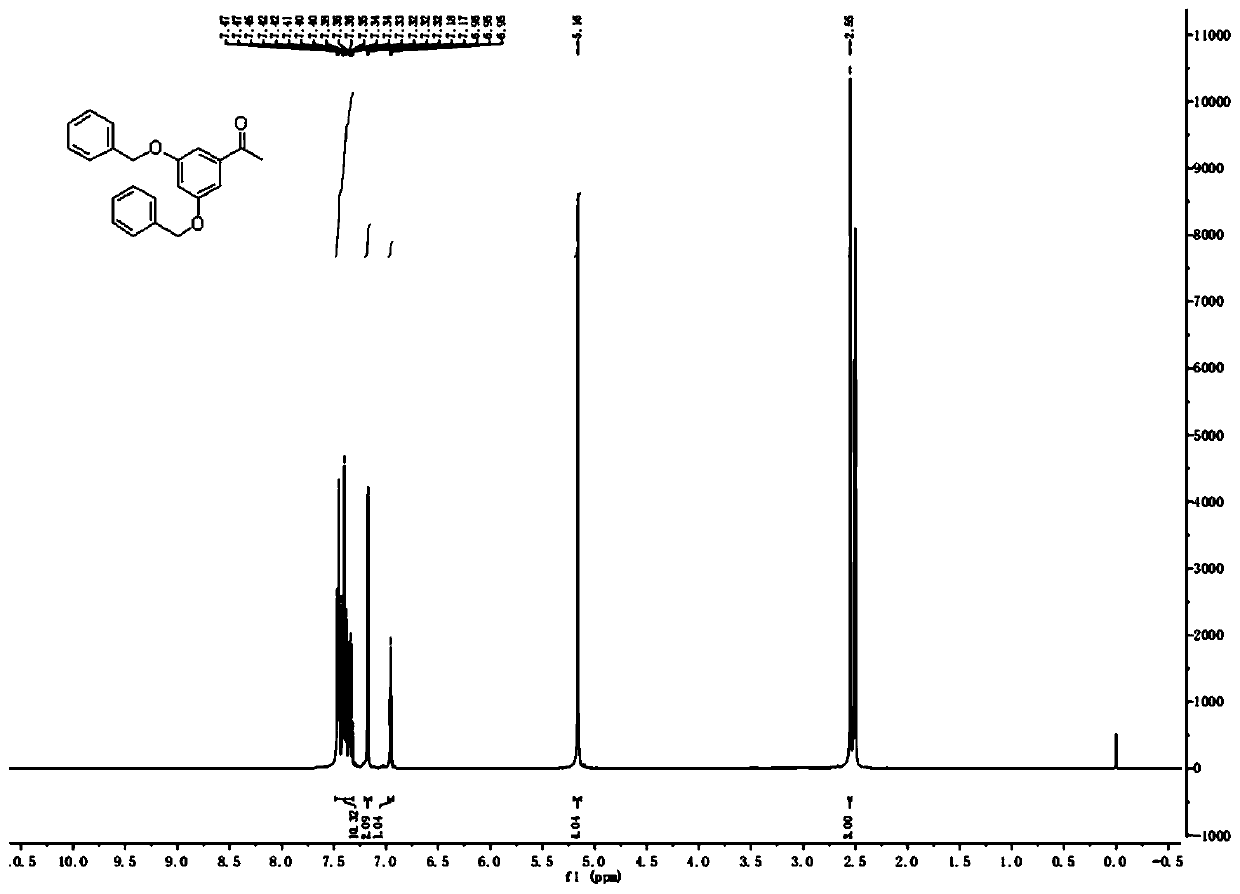
[0035] At room temperature, 100.0g (657.25mmol) of 3,5-dihydroxyacetophenone, 227.1g (1.64mol) of anhydrous potassium carbonate, 1L of acetone and 174.7g (1.38mol) of benzyl chloride were successively added to the reaction vessel. , Reacted at 50°C for 6h. The reaction solution was cooled to room temperature, filtered with suction, the filter cake was washed with acetone (2×100mL), the combined filtrate was evaporated under reduced pressure to remove the acetone, and the residue was recrystallized with methanol to obtain an off-white solid, namely 3,5-dibenzyloxybenzene Ethanone 175.0g, yield 80%.
[0036] 1 H NMR (300MHz, DMSO-d6): δ=7.32-7.47(m, 10H), 7.18(d, J=1.8Hz, 2H), 6.96(t, J=1.8Hz, 1H), 5.16(s, 4H ),2.55(s,3H).
[0037] (2) Preparation of 3,5-dibenzyloxyacetophenone aldehyde
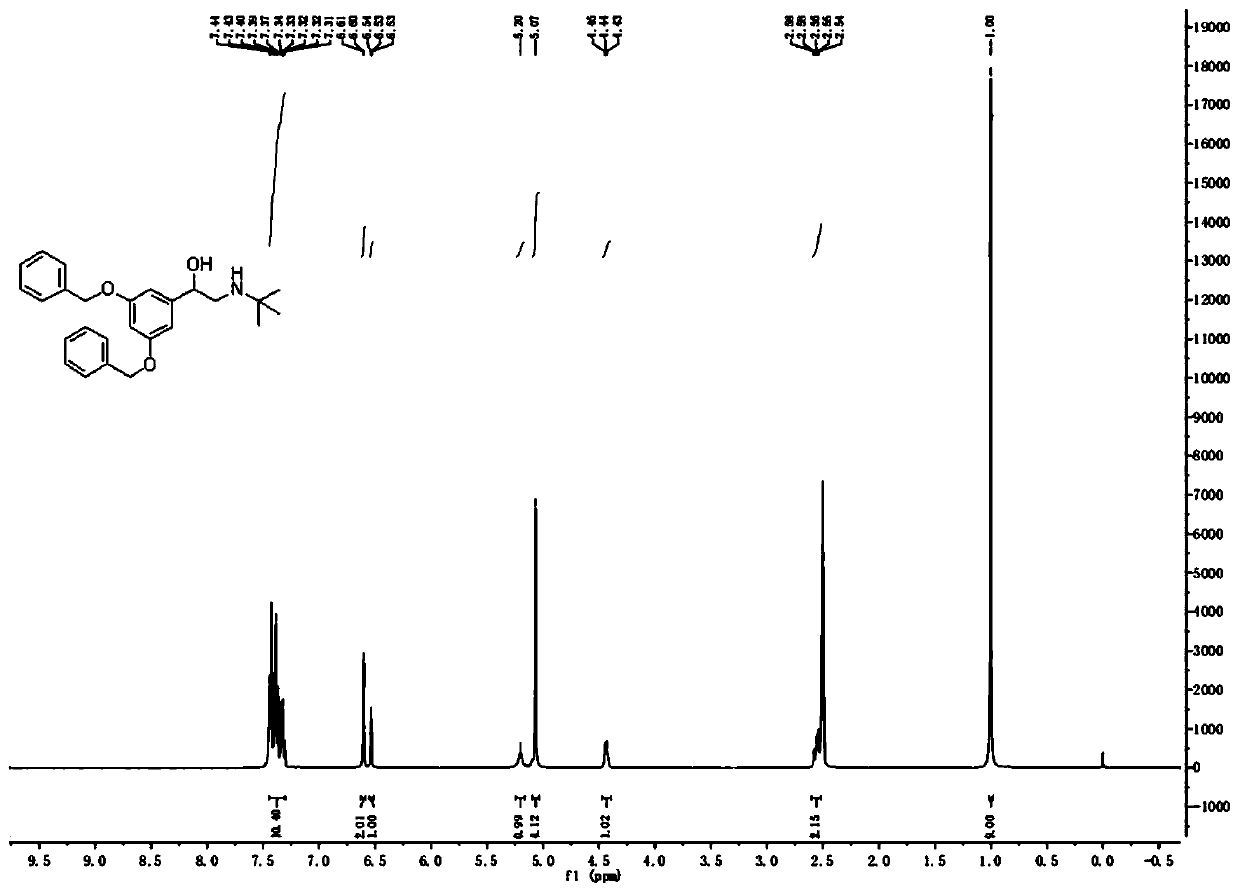
[0038]At room temperature, add 100.0g (300.84mmol) 3...
Embodiment 2
[0048] A preparation method of terbutaline sulfate, specifically comprising:
[0049] (1) Preparation of 3,5-dibenzyloxyacetophenone
[0050] At room temperature, 150.0g (985.88mmol) of 3,5-dihydroxyacetophenone, 313.4g (2.27mol) of anhydrous potassium carbonate, 1L of acetone and 262.1g (2.07mol) of benzyl chloride were successively added to the reaction vessel, and the addition was completed. , React at 60°C for 4h. The reaction solution was cooled to room temperature, filtered with suction, the filter cake was washed with acetone (2×100mL), the combined filtrate was evaporated under reduced pressure to remove the acetone, and the residue was recrystallized with methanol to obtain an off-white solid, namely 3,5-dibenzyloxybenzene Ethanone 268.7g, yield 82%.
[0051] 1 H NMR (300MHz, DMSO-d6): δ=7.32-7.47(m, 10H), 7.18(d, J=1.8Hz, 2H), 6.96(t, J=1.8Hz, 1H), 5.16(s, 4H ),2.55(s,3H).
[0052] (2) Preparation of 3,5-dibenzyloxyacetophenone aldehyde
[0053] At room tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com