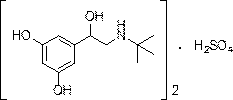
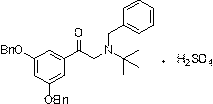
A kind of preparation method of terbutaline sulfate
A technology of terbutaline sulfate and concentrated sulfuric acid, which is applied in the field of medicine and chemical industry, can solve the problems of increased post-processing cost and environmental protection pressure of mixed solvents, high market price of raw materials, poor purification effect, etc., to avoid high energy consumption operating conditions The use of water, avoiding the introduction of water and inorganic salts, and reducing the effect of environmental protection pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]Add 1.18kg of the compound of formula I to 13.8L of glacial acetic acid and 59g of palladium carbon, replace with nitrogen 6 times, hydrogen 6 times, and stir at 60°C under normal pressure for 5 hours in the dark. Cool down to 10-15°C, suction filter under nitrogen atmosphere, place the suction filtrate in a reaction flask, add acetic acid solution of concentrated sulfuric acid equivalent to 0.5eq of the compound of formula I (mass volume concentration is 5%) under nitrogen protection, stir for 15min, Transfer to a distillation flask, replace with nitrogen, distill under reduced pressure, concentrate, cool down to room temperature, add 650ml of methanol, stir and crystallize under nitrogen atmosphere for 30min, drop to 10-15℃ for 1.5h, suction filter to obtain a white crystalline solid 645g, yield 98.4%, purity 99.87%.
Embodiment 2
[0045] Add 1.19 kg of the compound of formula II to 17.8 L of glacial acetic acid and 119 g of Ni, replace with nitrogen 6 times and hydrogen 6 times, and stir at 50°C under normal pressure for 4 hours in the dark. Cool down to 10-15°C, suction filter under a nitrogen atmosphere, place the suction filtrate in a reaction flask, add an acetic acid solution of concentrated sulfuric acid equivalent to 0.505 eq of the compound of formula I (mass volume concentration: 8%) under nitrogen protection, and stir for 20 minutes. Transfer to a distillation flask, replace with nitrogen, distill under reduced pressure, concentrate, cool down to room temperature, add methanol, stir and crystallize under a nitrogen atmosphere for 30 minutes, drop to 10-15°C for 2 hours, and filter with suction to obtain 652 g of a white crystalline solid. Yield 99.1%, purity 99.83%.
Embodiment 3
[0047] Add 1.18kg formula I compound to 11.8L glacial acetic acid and 59g Pd(OH) 2 , 6 times of nitrogen replacement, 6 times of hydrogen replacement, stirring at 40° C. under normal pressure in the dark for 5 h. Cool down to 10-15°C, suction filter under nitrogen atmosphere, place the suction filtrate in a reaction flask, add acetic acid solution of concentrated sulfuric acid equivalent to 0.495eq of the compound of formula I (mass volume concentration is 5%) under nitrogen protection, and stir for 15min. Transfer to a distillation flask, replace with nitrogen, distill under reduced pressure, concentrate, cool down to room temperature, add 1300ml of methanol, stir and crystallize under nitrogen atmosphere for 30min, drop to 10-15°C for 1.5h, and suction filter to obtain a white crystalline solid 642g, yield 98.0%, purity 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com