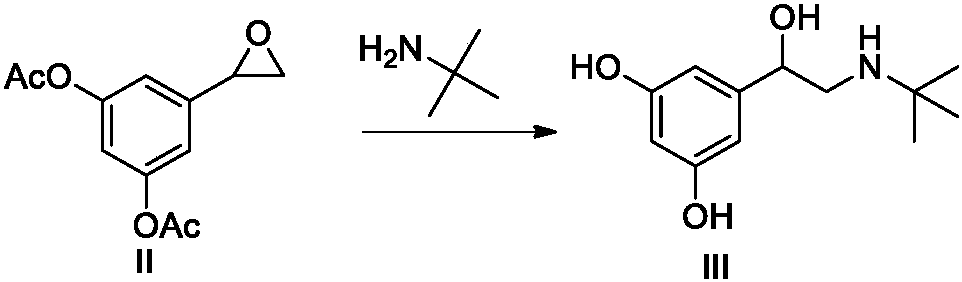
Novel preparation method of terbutaline sulfate
A technology of terbutaline sulfate and terbutaline, which is applied in the field of pharmaceutical technology, can solve problems such as danger and complicated hydrogenation debenzylation operation, and achieve the effect of cheap reagents and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
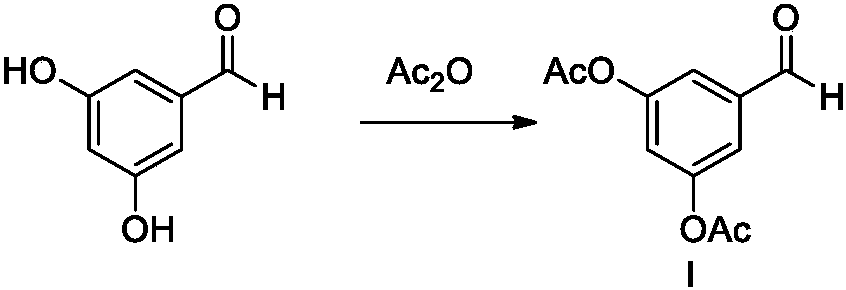
[0040] (1) Add 3,5-dihydroxybenzaldehyde (138g, 1.0mol) and dichloromethane (500mL) to a 1L reaction kettle, stir to dissolve, add acetic anhydride (190mL, 2.0mol), and cool to 0℃ Anhydrous aluminum trichloride (267g, 2.0mol) was added in batches, the reaction temperature was controlled between 0-10°C, the addition was completed within 1 hour, and the reaction was conducted at 5-10°C for 2 hours. After the reaction is completed, slowly pour into ice water (300mL), stir for 30min, separate the layers, wash the organic phase with water (300mL) and concentrate under reduced pressure at 40°C to obtain a light yellow-brown solid, and dry under reduced pressure at 40°C to obtain compound I (180g) , Yield 81.0%).
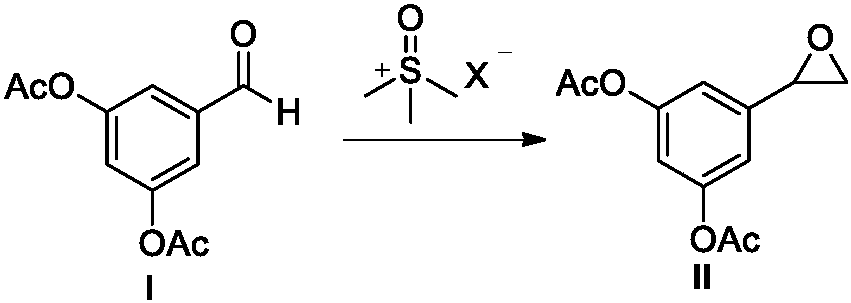
[0041] (2) Add trimethyl thionyl chloride (141g, 1.1mol), potassium tert-butoxide (124g, 1.1mol) to a 3L reaction kettle, and then add compound I (222g, 1.0mol) dimethylsulfoxide The sulfone (1L) solution was stirred to dissolve and then stirred for another 20 minutes, then ...
Embodiment 2
[0045] (1) Add 3,5-dihydroxybenzaldehyde (138g, 1.0mol) and dichloromethane (500mL) to a 1000ml reactor, stir to dissolve, add acetic anhydride (217mL, 2.3mol), and cool to 10°C Anhydrous aluminum trichloride (307g, 2.3mol) was added in batches, the reaction temperature was controlled between 20-30°C, the addition was completed within 1 hour, and the reaction was conducted at 20-30°C for 2 hours. After the reaction is completed, slowly pour into ice water (300mL), stir for 30min, separate the layers, wash the organic phase with water (300mL) and concentrate under reduced pressure at 40°C to obtain a light yellow-brown solid, and dry under reduced pressure at 40°C to obtain compound I (198g) , The yield is 89.1%).
[0046] (2) Add trimethyl sulfoxide bromide (260g, 1.5mol), potassium tert-butoxide (168g, 1.5mol) to a 3L reaction kettle, and then add compound I (222g, 1.0mol) dimethylsulfoxide The sulfone (900 mL) solution was stirred for dissolution and then stirred for another 2...
Embodiment 3
[0050] (1) Add 3,5-dihydroxybenzaldehyde (138g, 1.0mol) and dichloromethane (500mL) to a 1000ml reaction kettle, stir to dissolve, add acetic anhydride (236mL, 2.5mol), and cool to 10℃ Anhydrous aluminum trichloride (333g, 2.5mol) was added in batches, the reaction temperature was controlled between 10-20°C, the addition was completed within 1h, and the reaction was carried out at 10-20°C for 2h. After the reaction is completed, slowly pour into ice water (300mL), stir for 30min, separate the layers, wash the organic phase with water (300mL) and concentrate under reduced pressure at 40°C to obtain a light yellow-brown solid, and dry under reduced pressure at 40°C to obtain compound I (182g) , 81.9%).
[0051] (2) Add trimethyl sulfoxide iodide (264g,), potassium tert-butoxide (135g, 1.2mol) to a 3L reaction kettle, and then add compound I (222g, 1.0mol) of dimethyl sulfoxide ( 600mL) solution, stirred for dissolution and then stirred for another 20min, then heated to 50°C and co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com