Pharmaceutical polymer composition for oral controlled-release delivery of terbutaline sulfate
A technology of terbutaline sulfate and controlled-release drugs, which is applied in the field of sodium bicarbonate, and can solve problems such as slowing down of outflow and delayed release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1: Study on Controlled Release Performance of Polymer Combination
[0088] Preparation of controlled-release tablets
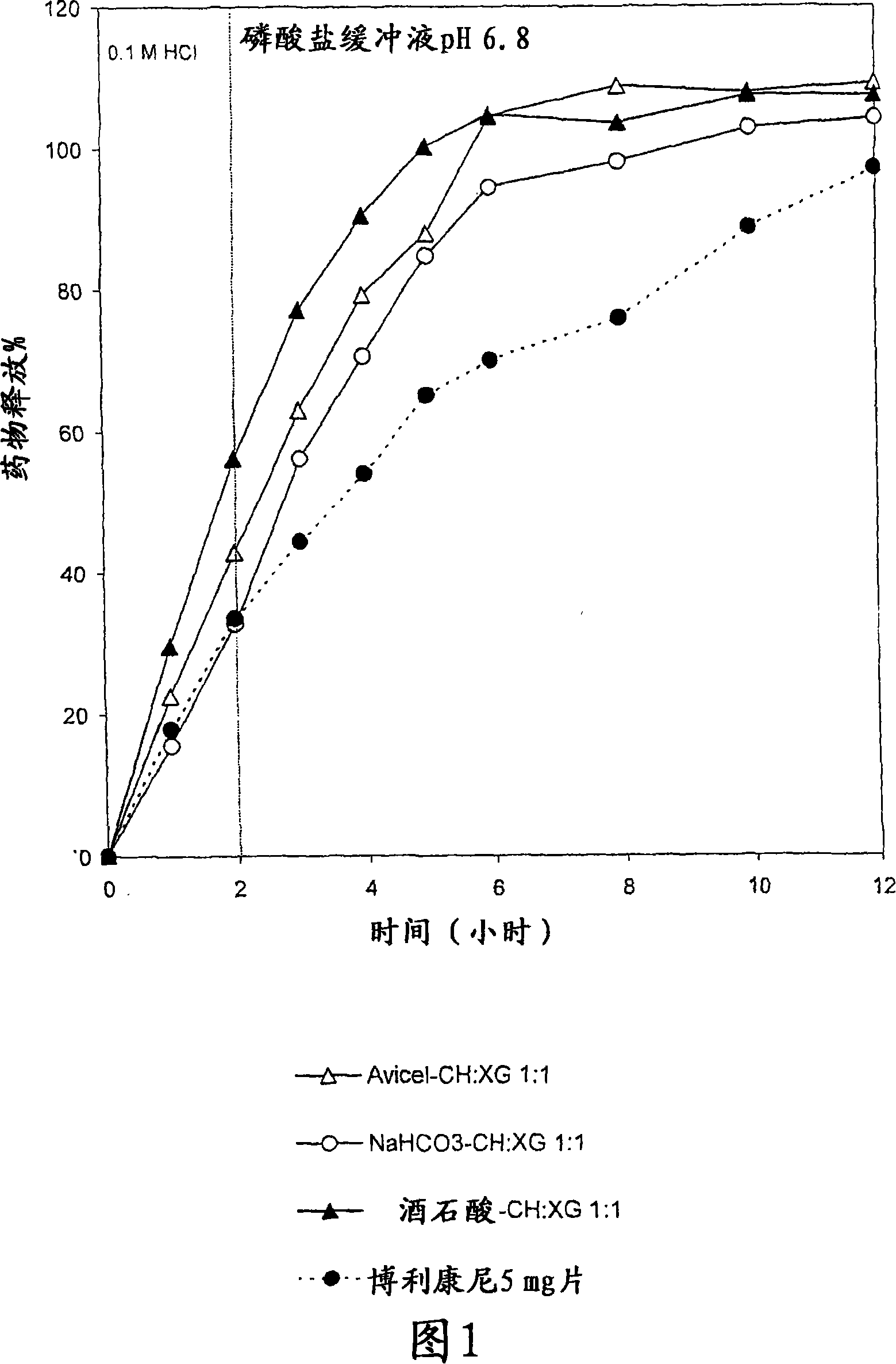
[0089] The system contained 5 mg terbutaline sulfate and 15 mg release modifying excipient (acidic: tartaric acid, or basic: sodium bicarbonate, or neutral: Avicel PH101 ) and 60 mg release delaying polymer in each single matrix. The polymer mixture was CH:XG 1:1 (w / w). The ratio of drug / excipient to polymer mixture was 1:3 (see Table 1). Tablets of 7 mm diameter were prepared by direct compression. The ingredients for each tablet were geometrically mixed using a porcelain mortar and pestle for about 10 minutes prior to pressing. Biplanar tablets were prepared by compressing the powder mixture with a hydraulic press applying a pressure of about 443 MPa for 15 seconds.
[0090] In Vitro Dissolution Test
[0091] USP Apparatus 1 (basket) was used. During the test the containers were placed in a water bath adjusted to maintain a temperat...
Embodiment 2
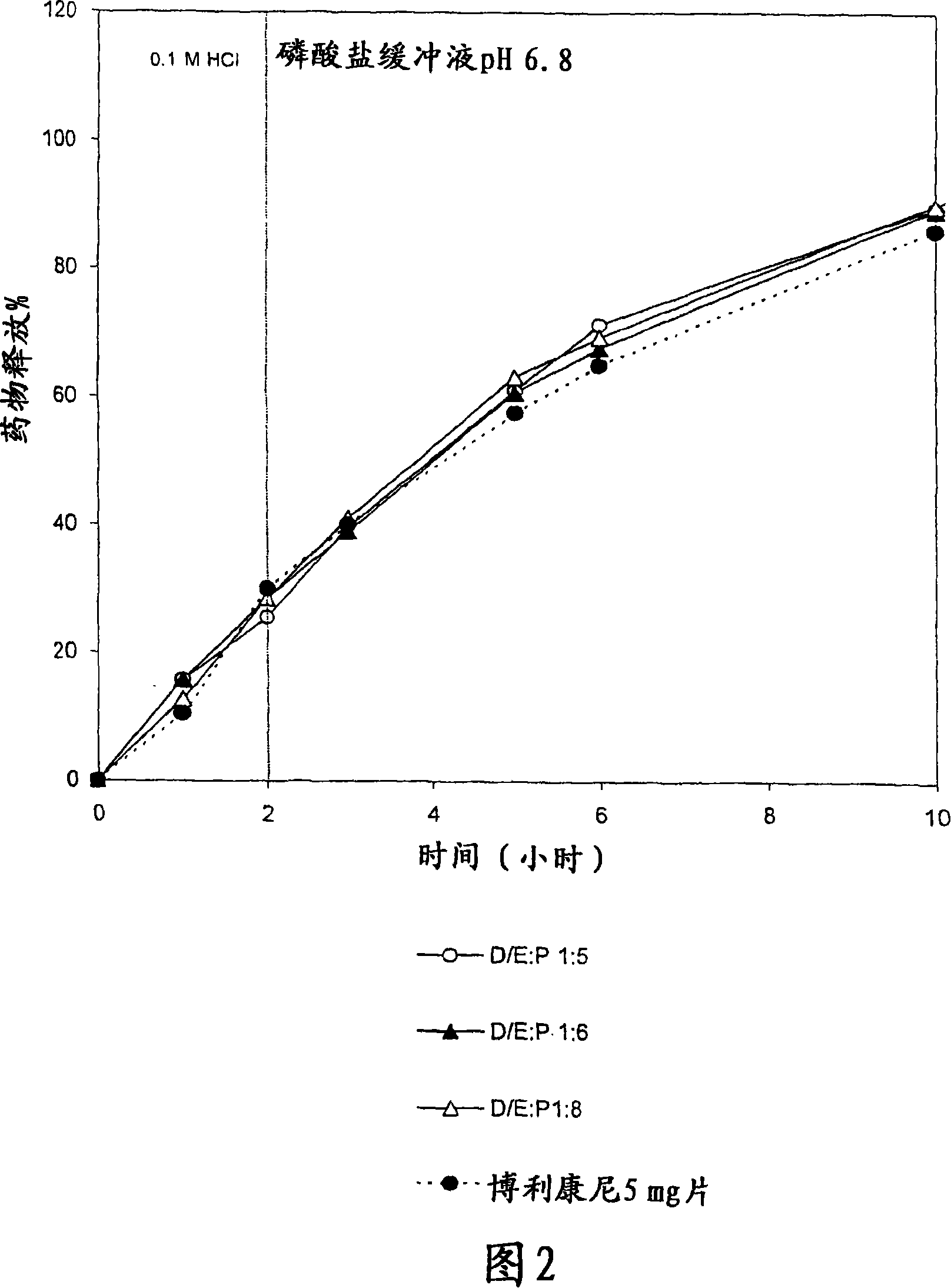
[0104] Example 2: Sieve out polymer mixture (P) (chitosan / xanthan gum 1:1) and carbon Suitable D / E:P ratio of sodium bicarbonate excipient (E)
[0105] The system contained 5 mg terbutaline sulphate and 15 mg release modifying excipient (sodium bicarbonate) and release delaying polymer mixture. The polymer mixture is CH:XG in a 1:1 ratio. The drug / excipient to polymer mixture (D / E:P) ratios were 1:5, 1:6 and 1:8, as shown in Table 4. Tablets of 7 mm diameter were prepared by direct compression. The ingredients for each tablet were geometrically mixed using a porcelain mortar and pestle for about 10 minutes prior to pressing. Biplanar tablets were prepared by compressing the powder mixture with a hydraulic press applying a pressure of about 443 MPa for 15 seconds. The in vitro dissolution conditions and analysis methods are similar to those mentioned in Example 1.
[0106] Table 4 : Overview of the formulation used in the development of a controlled-release product of...
Embodiment 3
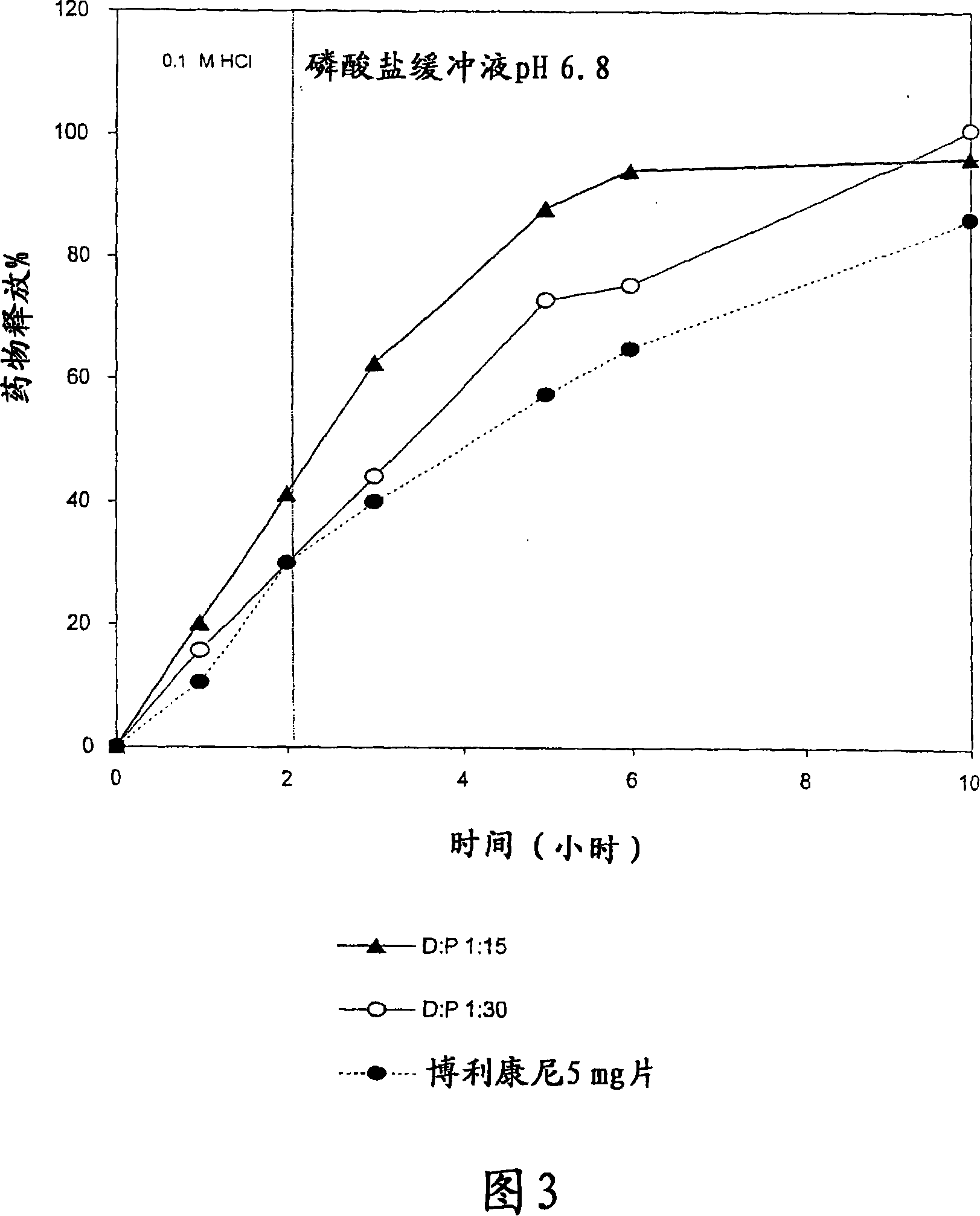
[0114] Example 3: Powders with a D / E:P ratio of 1:5 were directly compressed by using a single-punch tablet press small-scale experiment
[0115] The system contains 5 mg Terbutaline Sulfate and 15 mg release modifying excipient (sodium bicarbonate) and release delaying polymer blend in each tablet. The polymer mixture was CH:XG in a 1:1 ratio. The drug / excipient to polymer mixture (D / E:P) ratio was 1:5 (see Table 6). The ingredients for each tablet were geometrically mixed for about 10 minutes prior to compression. Biplanar tablets of 7 mm diameter were prepared by direct compression method using a single-punch tablet press. The batch size was 500 g and the batch number was called S1. The proposed generic designations for Lot S1 tablets are "Talin" and "Talin Extended Release Tablets" and "Talin XR". Five tablets were randomly selected from Bricanyl Durules and Talin XR for physical characterization (see Tables 7 and 8, respectively). The in vitro dissolution conditi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com