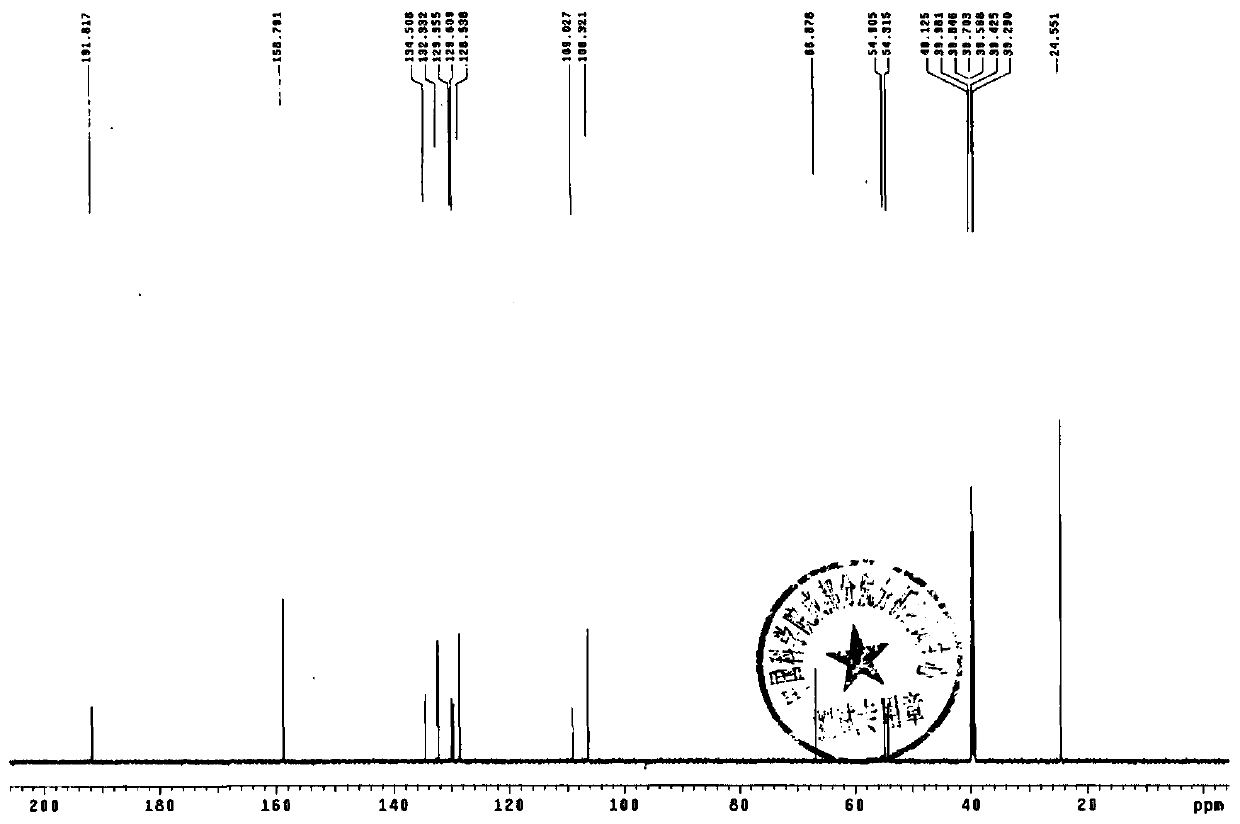
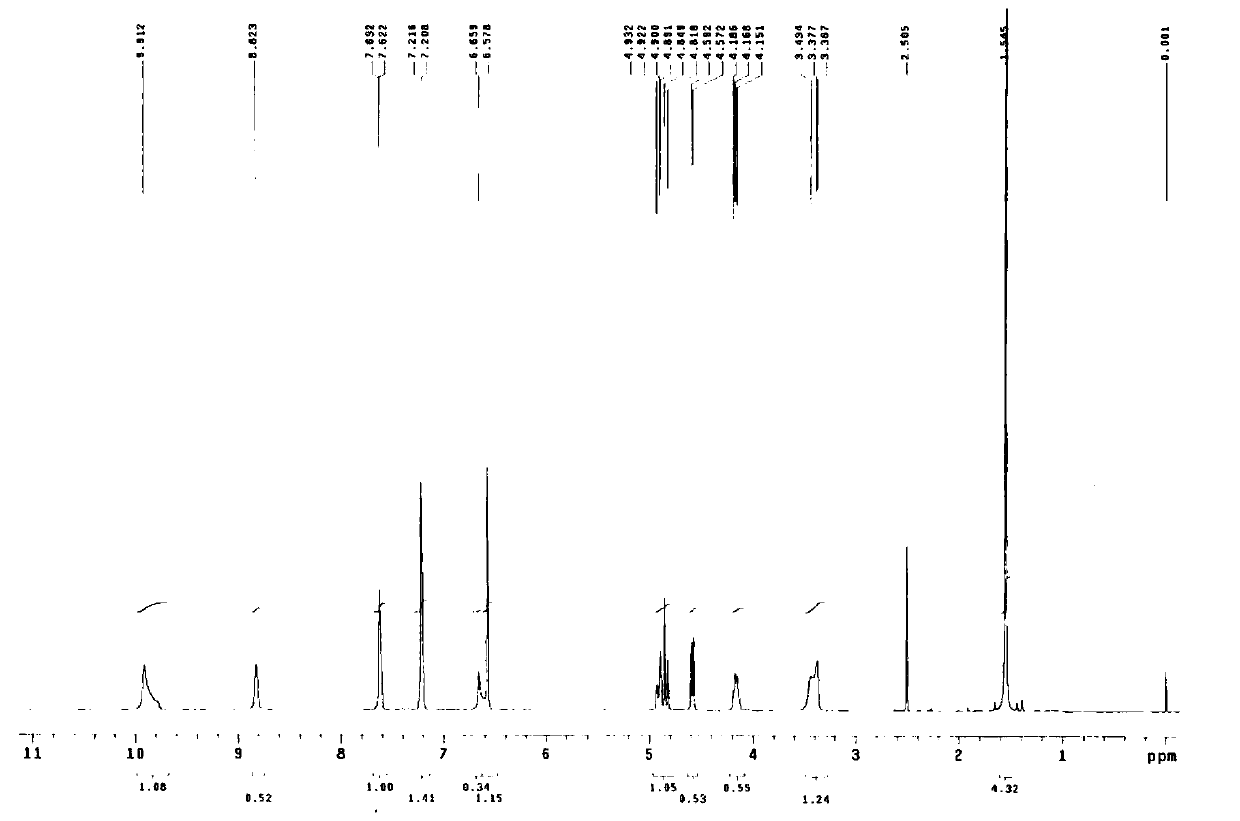
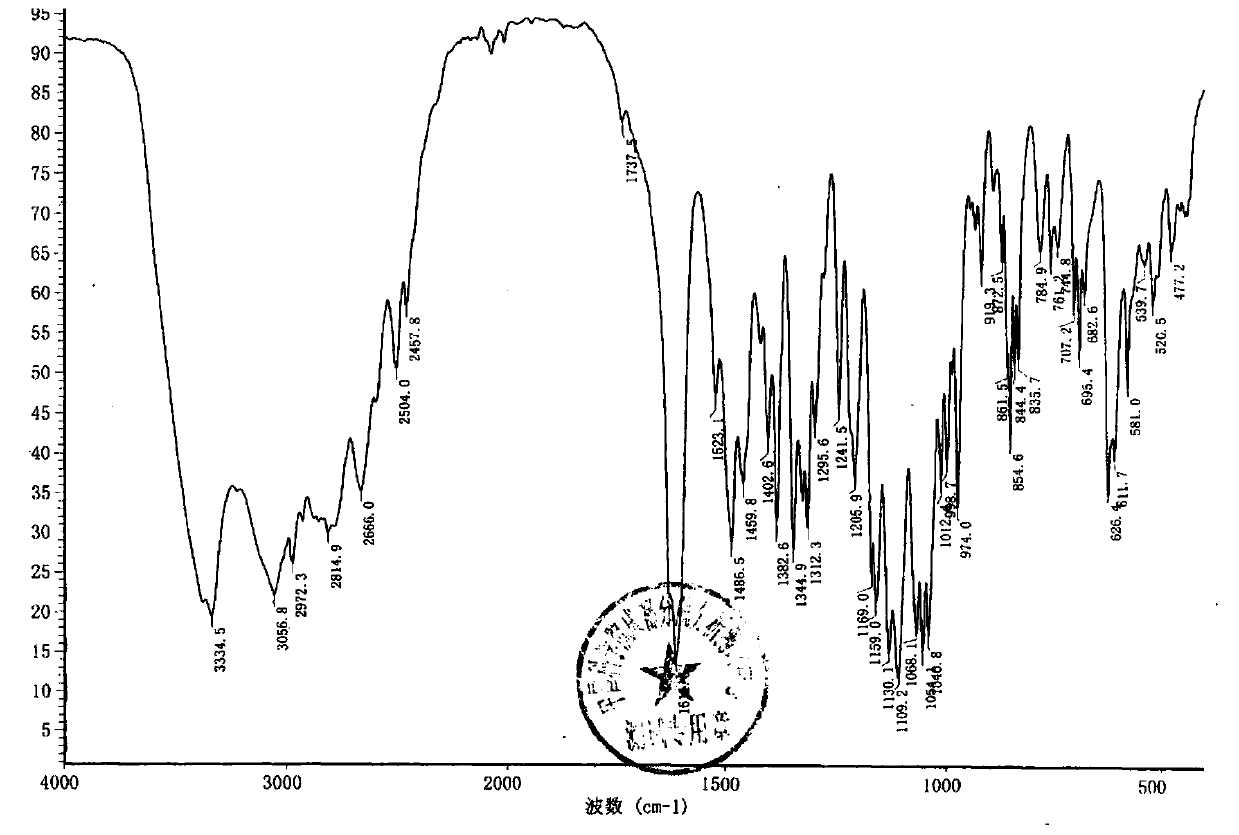
Terbutaline sulfate intermediate, preparation method thereof, and method for preparing terbutaline sulfate from terbutaline sulfate intermediate
A technology for terbutaline sulfate and intermediates, applied in the field of medicinal chemistry, can solve problems such as difficulty in extracting terbutaline free base, decline in product quality and yield, unstable free base quality, etc., to avoid product quality and yield reduction, short reaction time and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1: preparation compound 2 (R' is acetyl group)
[0084] Add 76mL of dichloromethane and 38g of 3,5-diacetoxyacetophenone into the reaction flask with tail gas absorption device, after stirring and dissolving completely, add dropwise a few drops of bromine-dichloromethane mixed solution (bromine element 27g dichloromethane 27ml), insulated and stirred until acid gas (checked with wet pH test paper) escaped and cooled to below 10°C, kept warm and continued to add bromine-dichloromethane solution dropwise, and the spots of the raw materials monitored by TLC basically disappeared ( TLC monitoring method: Silicone GF 254 , Developing agent: dichloromethane-ethyl acetate (10:1), ultraviolet lamp 254nm color), stop the reaction. The reaction solution was washed with water until the pH of the aqueous layer was 6-7, the dichloromethane layer was dried with anhydrous magnesium sulfate, filtered, and the solvent was evaporated under reduced pressure, and the residue wa...
Embodiment 2
[0085] Embodiment 2: preparation compound 2 (R' is acetyl group)
[0086] Add 236g of 3,5-diacetoxyacetophenone and 500mL of dichloromethane into a reaction flask with a tail gas absorption device, stir and dissolve completely, then add a few drops of a solution of 160g of bromine and 160ml of dichloromethane at room temperature, Insulate and stir until hydrogen bromide gas (check with wet pH test paper) escapes, then cool down to below 10°C, continue to add bromine dichloromethane solution dropwise, and the spots of raw materials monitored by TLC basically disappear (thin-layer monitoring method: silica gel GF 254 , Developing agent: dichloromethane-ethyl acetate (10:1), UV lamp 254nm color development), washed with water to pH 6-7, dried the organic layer with anhydrous magnesium sulfate, filtered, removed the solvent under reduced pressure, and added the residue to Crystallization from absolute ethanol yielded compound 2 with a yield of 246 g and a yield of 78%.
Embodiment 3
[0087] Embodiment 3: preparation compound 2 (R' is acetyl group)
[0088] Add 236g of 3,5-diacetoxyacetophenone and 472mL of dichloromethane into the reaction flask with tail gas absorbing device. After stirring and dissolving completely, add a few drops of 176g of bromine and 176mL of dichloromethane solution under stirring at room temperature. After hydrogen bromide gas (check with wet pH test paper) escapes, lower the temperature to below 10°C, continue to add bromodichloromethane solution dropwise, and the spots of the raw material will basically disappear under TLC monitoring (thin-layer monitoring method: silica gel GF 254 , Developing agent: dichloromethane-ethyl acetate (10:1), ultraviolet lamp 254nm color development), nitrogen or air to remove hydrogen bromide, water washing to pH6~7, organic layer dried over anhydrous magnesium sulfate, filtered, The solvent was removed under reduced pressure, and the residue was crystallized by adding absolute ethanol to obtain com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com